Abstract
The posterior mediastinum is a rare site of origin for seminoma, and extensive cystic change of a seminoma is rarely observed. We present here the case of a 62-year-old male patient with a primary posterior mediastinal seminoma that was encasing the descending thoracic aorta and the tumor showed prominent cystic change on CT and MRI, and intense FDG uptake on PET/CT.
Seminoma is the most common histologic subtype of malignant germ cell tumors, and it most frequently occurs in the anterior mediastinum in the chest (1). The posterior mediastinum is a rare site of origin for seminoma, and prominent cystic change of a posterior mediastinal seminoma has not been previously described. The present report describes the CT, MR and 18F-FDG PET/CT findings of a case of a primary posterior mediastinal seminoma that was encasing the descending thoracic aorta and the tumor displayed prominent cystic change.
A 62-year-old man who complained of back pain for the previous 2 months was referred to our hospital with a posterior mediastinal mass. Chest CT revealed a huge posterior mediastinal mass enveloping the descending thoracic aorta and displacing the heart anteriorly, and also an enlarged left peribronchial lymph node. The mass was well defined with an extensive low attenuation area and periaortic soft tissue components (Fig. 1A). Metastatic bone destruction was noted in the T7-T11 vertebrae. The chest CT obtained at an outside clinic 40 days before admission showed that the cystic area of the mass had heterogeneous high density (31-46 HU) on both the pre- and post-contrast-enhanced imaging. T2-weighted MRI of the chest showed the periaortic soft tissue components and the predominantly cystic nature of the mass (Fig. 1B). The mass displayed heterogeneously low intensity on the T1-weighted image. 18F-FDG PET/CT revealed marked uptake in the periaortic area and in some of the peripheral portion of the mass, and in a peribronchial lymph node (maximum SUV: 5.8-7.9) on the 1-hour post-injection image (Figs. 1C, D). The percutaneous aspirate of the cystic component contained blood and necrotic debris that was suggestive of previous hemorrhage and necrosis within the tumor. Our initial working diagnosis was lymphoma or metastatic cancer with periaortic hematoma formation. Thoracoscopic biopsy was performed to obtain sufficient tissue and a diagnosis of seminoma was made. Blood tests for germ cell tumor markers showed normal levels of alpha-fetoprotein and human chorionic gonadotropin (hCG). The serum L-lactate dehydrogenase (LDH) level was elevated to 502 IU/L (normal: 135-225 IU/L). Testicular sonography was performed to exclude a primary testicular tumor. The testes were normally positioned and symmetric in size without mass lesions. After the diagnosis, the physician intended to perform prompt chemotherapy, but the patient refused any treatment and he was discharged.
The patient visited the emergency room with paraplegia 1 month later. MRI of the spine revealed multiple metastatic lesions in the T7-T11 vertebrae and pathologic compression fractures of the T9 and T10 vertebral bodies with cord compression. The patient expired after a 2 week bout of sepsis following one cycle of a standard BEP chemotherapy regimen (30 mg of bleomycin for 1 day every week, 100 mg/m2/day of etoposide and 20 mg/m2/day of cisplatin on Days 1-5, every 3 weeks).
Primary mediastinal germ cell tumors account for only 1-15% of all the mediastinal masses in adults. Mediastinal seminoma is a rare neoplasm that primarily affects men in their third or fourth decade of life (1). Hormonal activity is not usually apparent in seminoma.
It was previously thought that mediastinal malignant germ cell tumors represent metastatic spread from an occult gonadal primary neoplasm; however, the results from autopsy studies have demonstrated that germ cell tumors can occur as primary mediastinal neoplasms in the absence of a testicular tumor (2). The incidence of metastasis to the mediastinum from testicular seminoma is rare (1).
Germ cells can be misplaced in the posterior mediastinum because this area is located in the migration tract during embryogenesis, from the anterior mediastinum to the retroperitoneum, yet germ cell tumors arising in the posterior mediastinum are extremely rare (13). In the present case, the mass was located in the posterior mediastinum and it encased the descending thoracic aorta. Although other germ cell neoplasms have been described in the posterior mediastinum (4), to the best of our knowledge, a primary posterior mediastinal seminoma has been previously described only twice in the English medical literature (35). Except for the presence of cystic change in the present case, these reports describe imaging appearances that are similar to those in our case, with a confluent posterior mediastinal mass encasing the descending thoracic aorta. Further, the patients in the 2 previously reported cases were older than the commonly affected age group, the same as our case.
Making the correct differential diagnosis between seminoma and nonseminomatous germ cell tumors or other malignancy is important because pure seminomas respond well to radiation or cisplatin-based chemotherapy, and the prognosis is favorable in the early stage of disease (6).
Radiologically, mediastinal seminomas present as bulky, well-circumscribed masses that extend to both sides of the midline. On CT, mediastinal seminomas typically have homogeneous soft tissue attenuation. In contrast to the appearance of nonseminomatous germ cell tumors on CT, it is well known that seminomas show few areas of low density (4). On the T2-weighted MR images, seminomatous tumors are hypointense and relatively homogeneous, which reflects their high cellularity (7). Prominent cystic change in a posterior mediastinal seminoma has not been previously reported. The present case showed extensive cystic change.
Flynn MJ et al. reported 3 cases of testicular seminoma with unusual cystic change (8). Two of those cases showed grossly prominent cystic change and the histology revealed cystic change within the cells of a syncytiotrophoblastic origin, and the serum hCG level was markedly elevated. In the present case, there was no other germ cell component in the biopsy specimen, and the hCG level was within the normal range. Moran et al. reported the pathologic findings of 10 cases of anterior mediastinal seminoma with prominent cystic change and these mimicked multilocular thymic cyst (9). Kurosaki et al. reported the CT findings and pathologic features of a case of anterior mediastinal seminoma with prominent cystic change (10). The microscopic features of the cystic structures were identical to those of multilocular thymic cysts, and nodules of seminoma were found in the cyst walls. The authors postulated that cystic change in anterior mediastinal seminoma represents an exaggerated hyperplastic response of the thymic epithelium to neoplasm. The pathogenesis of the cystic process in the posterior mediastinal seminoma may be different from that in the anterior mediastinal seminoma or testicular seminoma because there is no thymic tissue in the posterior mediastinum. In the present case, the CT obtained at an outside clinic 40 days before admission showed heterogeneously high density of the cystic area on the pre-contrast images (31-46 HU) and there was no contrast enhancement post-injection, which indicated hemorrhage. Percutaneous aspiration of the cystic area showed hemorrhagic necrosis.
In summary, we report here on a posterior mediastinal seminoma that showed unusually predominant cystic change due to hemorrhage and necrosis. Seminoma should be considered in the differential diagnosis of posterior mediastinal masses that encircle the descending thoracic aorta, and even in older males.
Figures and Tables
Fig. 1
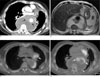
A posterior mediastinal seminoma with prominent cystic change in a 62-year-old man.
A. The contrast-enhanced chest CT scan shows a large cystic posterior mediastinal mass enveloping the descending thoracic aorta. Soft tissue components are present in the periaortic area (arrows). The attenuation coefficients of the cystic area ranged from 1 to 19 HUs on the unenhanced and enhanced scans.
B. The axial T2-weighted MR imaging of the chest shows the extensive cystic and periaortic soft tissue components of the mass.
C. 18F-FDG PET/CT at the level of the carina shows intense uptake by a peribronchial lymph node (arrow) and in the periaortic area.
D. 18F-FDG PET/CT at the level of the left atrium shows hypermetabolic components in the periaortic area (arrows), the periphery
of the mass (arrowhead) and the thoracic spine.
References
1. Moran CA, Suster S, Przygodzki RM, Koss MN. Primary germ cell tumors of the mediastinum: II. Mediastinal seminomas -- a clinicopathologic and immunohistochemical study of 120 cases. Cancer. 1997; 80:691–698.
2. Luna MA, Valenzuela-Tamariz J. Germ-cell tumors of the mediastinum, postmortem findings. Am J Clin Pathol. 1976; 65:450–454.
3. Ravenel JG, Gordon LL, Block MI, Chaudhary U. Primary posterior mediastinal seminoma. AJR Am J Roentgenol. 2004; 183:1835–1837.
4. Rosado-de-Christenson ML, Templeton PA, Moran CA. From the archives of the AFIP. Mediastinal germ cell tumors: radiologic and pathologic correlation. Radiographics. 1992; 12:1013–1030.
5. Makiyama K, Senga Y. Primary seminoma in the posterior mediastinum. J Urol. 2001; 165:908.
6. Bokemeyer C, Droz JP, Horwich A, Gerl A, Fossa SD, Beyer J, et al. Extragonadal seminoma: an international multicenter analysis of prognostic factors and long term treatment outcome. Cancer. 2001; 91:1394–1401.
7. Johnson JO, Mattrey RF, Phillipson J. Differentiation of seminomatous from nonseminomatous testicular tumors with MR imaging. AJR Am J Roentgenol. 1990; 154:539–543.
8. Flynn MJ, Childerhouse A, Mead GM, Theaker JM. Unusual cystic change in classic seminoma of the testis. Am J Surg Pathol. 2006; 30:137–139.
9. Moran CA, Suster S. Mediastinal seminomas with prominent cystic changes. A clinicopathologic study of 10 cases. Am J Surg Pathol. 1995; 19:1047–1053.
10. Kurosaki Y, Tanaka YO, Itai Y. Thymic seminoma with prominent cystic changes. AJR Am J Roentgenol. 1996; 167:1345–1346.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download