Abstract
The Carney complex, the first reported in a series of 40 patients by J. A. Carney in 1985, is an autosomal dominant disease that is characterized by multiple neoplasia. The disease has a familial pattern in approximately one-half of the patients. We present the three cases of the Carney complex within one family.
The Carney complex is a multiple familial neoplastic disorder characterized by spotty pigmentation of the skin and mucosa, myxomatous tumors of the skin, heart, breasts, other organs, various endocrine tumors with or without endocrine overactivity, testicular tumors (large-cell calcifying Sertoli and Leydig cell tumors), psammomatous melanotic schwannomas, and thyroid follicular tumors (1). The disease is inherited in an autosomal dominant manner, but can also occur sporadically. More than 400 cases have described the various clinical manifestations of the Carney complex, in addition to results of genetic studies related to the Carney complex. However, few radiologic findings associated with the Carney complex have been reported (1). Moreover, reports of the Carney complex in first-degree relatives are very rare.
Herein, we present three cases of the Carney complex in first-degree relatives, (a mother and her two sons). We describe the various components related to the Carney complex, as well as the radiologic findings, treatment, and clinical outcomes. In addition, through our experiences and the literature, we propose an algorithm for screening and a serial follow-up examination of patients with the Carney complex as well as their first-degree relatives.
A 12-year-old boy, the second son of the family, presented with generalized tonic-clonic type seizures in a comatose state. The seizures persisted for more than 30 minutes. The boy had no prior history of seizures. Upon physical examination, a cardiac murmur was auscultated.
To rule out an organic disease of the brain, a brain CT was obtained. The brain CT images showed subtle low attenuation in the left cerebellar hemispheres, which suggested an acute cerebral infarction. Diffusion-weighted and routine MR images were obtained for further evaluation, and revealed a multifocal acute embolic infarction that involved the cerebellar hemispheres, thalami, pons, midbrain, left basal ganglia, left hippocampus, the body and splenium of the corpus callosum, both cerebral cortices, and subcortical white matter (Figs. 1A, B). Based on the findings, which include young age, auscultation of the cardiac murmur, and multiple vascular territories on brain imaging, there was a high probability that the boy suffered a cardiogenic embolic infarction. A transesophageal echocardiography was thus performed to rule out a cardiogenic embolic source. The echocardiography revealed two echogenic masses attached to the left atrium. The boy subsequently underwent surgical removal of the cardiac tumor and a histopathologic examination showed a cardiac myxoma.
For evaluation of the abdominal lesions, we performed an abdomino-pelvic CT with contrast enhancement, which revealed solid lesions with multiple internal calcifications in both testes (Fig. 1C). A scrotal sonography showed multiple complex masses and variablesized multiple calcifications with heavy posterior acoustic shadowing in both testes. Each mass measured less than 2 cm. The tunica albuginea of the testis was preserved; however, evidence of a malignant tumor, such as extratesticular extension, hemorrhage, or necrosis, was not found. On color Doppler sonography, arterial blood flow was markedly increased around the nodules (Fig. 1D). Because the boy met the diagnostic criteria for the Carney complex, we regarded the testicular tumor as another neoplasm in the disease spectrum of the Carney complex. Thus, we concluded that the testicular tumor was a large cell calcifying Sertoli and Leydig cell tumor.
On laboratory testing, the boy's human growth hormone level was slightly elevated (6.1 ng/mL; normal range, 0 - 4 ng/mL), however other hormone levels including cortisol, TSH, β-hCG and AFP, were within normal ranges. Dynamic pituitary MR imaging showed heterogeneous signal intensity in the pituitary gland; however, no definite nodules were seen, which suggests a pituitary adenoma. Upon physical examination, the boy's height and weight were 178 cm and 73 kg, respectively. His physical condition exceeded the 99th percentile, compared to boys within the same age group.
The boy was admitted and treated over a 7-week interval, and his current mental status was alert. In addition, there were no complications related to the open cardiac surgery. However, the severe embolic infarction resulted in neurologic sequelae, with motor weakness involving the right upper and lower extremities, (motor grade III-IV). He is now undergoing rehabilitation in an attempt to achieve full recovery from his neurologic sequelae.
A 14-year-old boy, the eldest son of the family, sought screening for a cardiac lesion, because his brother (case 1) presented with an acute cerebral embolic infarction originating from a cardiac myxoma, a month earlier. We first performed an echocardiography and noted a 5 × 4 × 6 cm hyperechoic mass. The mass was attached to the left atrium and the movable portion protruded into the left ventricle, which caused mild mitral regurgitation. The other cardiac functions were found to be normal. To evaluate cardiac mass, a chest CT with contrast enhancement was obtained. The CT scan showed a low attenuation mass with a lobular shape and mild enhancement in the left atrium and left ventricle, suggestive of a cardiac myxoma (Fig. 2A). There was no evidence of a pulmonary embolism. The patient underwent surgical removal of the cardiac mass, and the histopathologic findings confirmed a cardiac myxoma (Fig. 2B).
A review of the patient's medical records revealed that he had, on two occasions, undergone an excisional biopsy of recurrent soft tissue lesions in the right external auditory canal at the ages of 11 and 13 years, respectively. The histopathologic findings described the masses as benign cystic lesions with abundant myxoid stroma.
An abdomino-pelvic CT with contrast enhancement was obtained for evaluation of other tumors. Several nodules were noted in both adrenal glands, the largest of which was 3.5 cm in maximum diameter. The largest nodule showed strong enhancement in the post-contrast images, and delayed wash-out after 10 minutes (Figs. 2C, D). The absolute percentage of wash-out was calculated to be 63%. An adrenal scintigraphy with 131I-labeled 6-beta-iodomethyl-19-norcholesterol (NP-59) was performed, which resulted in increased uptake in both adrenal glands, suggestive of functional adrenal cortical lesions. The laboratory data were consistent with ACTH-independent Cushing's syndrome.
On a pelvic CT scan, the left testis was shown to be asymmetrically enlarged, due to an enhancing mass with multiple calcifications, as had been noted in his brother.
Laboratory tests revealed elevated human growth hormone levels (10.8 ng/mL; normal range, 0 - 4 ng/mL). Therefore, dynamic MR imaging was performed for the evaluation of the pituitary gland. A brain MR showed multiple nodules with low signal intensity on T2WI and slight enhancement on the post-contrast image (Figs. 2E, F). The lesions were not confirmed pathologically, but were highly suggestive of pituitary adenomas. However, there was no gigantism.
A 38-year-old female, the mother of the family, presented with a palpable mass in the right breast.
Upon physical examination, skin pigmentations, similar to freckles, were scattered on her face, neck, and chest wall. Based on her medical records, she had undergone two open cardiac surgeries due to recurrent or metachronous myxomas in May 1999 and February 2001.
The initial mammography showed two nodular asymmetries in the upper portion and subareolar area of the right breast, in addition to an enlarged nodular lesion in the right axilla (Fig. 3A). A breast sonography showed multiple nodules in both breasts, which were categorized as BIRADS III lesions (Fig. 3B). In the right axilla, a lobulated nodular lesion with complex internal echogenicity and posterior acoustic enhancement was noted (Fig. 3C). We performed an ultrasound-guided core biopsy in the lesions of the right axilla and breast. Pathologically, the nodules of the right breast were shown to be a fibroadenoma with myxoid changes, and the lesion of the right axilla was confirmed to be a myxoma (Figs. 3D, E).
An abdominal CT with contrast enhancement was obtained for the evaluation of another tumor in the abdomen. We found a polypoid mass without definite enhancement in the right ventricle, which was a possible recurrent or metachronous cardiac myxoma. We performed an echocardiography, and the report was highly suggestive of a cardiac myxoma; however, surgical removal was not performed.
Since J.A. Carney reported a series of 40 patients with components of the Carney complex in 1985 (1), many case reports about the disease have been described. In addition, pedigree analyses of several large families affected by the Carney complex have demonstrated that the disease is transmitted in a Mendelian autosomal dominant pattern. Even though the incidence and epidemiology of the disease have not been well-established, the characteristic manifestations and genetics related to the Carney complex are known. Indeed, the disease is not only inherited as an autosomal dominant trait, but can also develop sporadically.
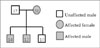
Although various clinical findings related to the Carney complex have been reported, case reports describing patients with the Carney complex in first-degree relatives are rare. These cases have clearly shown that the characteristic pattern is inherited in an autosomal dominant manner, from affected mother to her children, as well as the various systemic manifestations of the disease. The value of the current report lies in the clinical findings occurring in first-degree relatives. We have summarized the family pedigree and the clinical findings in Figure 4 and Table 1, respectively.
The Carney complex is a multiple familial neoplastic disorder that is characterized by cardiac myxomas, myxomatous tumors of the skin, breasts, and other organs, as well as spotty pigmentation of the skin and mucosa, various endocrine tumors, testicular tumors (large-cell calcifying Sertoli and Leydig cell tumors), psammomatous melanotic schwannomas, and thyroid follicular tumors (benign adenomas or carcinomas).
The Carney complex can be diagnosed when there is at least one characteristic clinical manifestation (vide supra) associated with the disease, and there is family history or at least two characteristic clinical findings in the absence of family history (2).
Cardiac myxomas are an important component of the Carney complex and occurred in 29 (72%) of the 40 patients in the initial series reported by Carney et al. (1). Cardiac myxomas in patients with the Carney complex tend to develop at a younger age than in patients with sporadic cardiac myxomas. In addition, cardiac myxomas in the Carney complex are more multicentric, and there is increased risk for recurrent and metachronous cardiac myxomas (34). Although these tumors are histologically benign, they can lead to obstruction of the cardiac outflow tract or systemic embolization and therefore, cause an elevated risk for morbidity and mortality. In our report, all three patients presented with cardiac myxomas, and a cardiogenic embolic infarction developed in one patient (case 1). The treatment of choice in cardiac myxomas is surgical removal. The risk of recurrent or metachronous myxoma is higher, thus a serial follow-up by echocardiography in patients with the Carney complex is essential. It is also important to screen the first-degree relatives. As depicted in the pedigree chart (Fig. 4), there are three sons in the family described herein. So, we performed echocardiography asymmetrically for screening of cardiac myxomas in the last son, which was normal.
Characteristic dermatologic findings are other important components of the Carney complex. Spotty skin pigmentations, so called lentigines and blue nevi, are the most common features in patients with the Carney complex. The face, (involving the eyelids, sclerae, lips, and cheeks), neck, trunk, and inguinal areas are the typically affected sites, but the effects can occur anywhere (1). As another dermatologic lesion, cutaneous myxomas are important features that need to be carefully assessed. Typical sites for cutaneous myxoma are the external auditory canal, eyelids, and nipples (1). In our case, the eldest son (case 2) presented with typical features of recurrent cutaneous myxomas that involved the right external auditory canals and both eyelids, which we subsequently treated by recurrent excisions.
Another myxomatous tumor occurs in the breast. It is known that the breast lesions in the Carney complex are myxoid fibroadenomas and ductal adenomas (15). Reports regarding the imaging findings of myxoid fibroadenomas and ductal adenomas are rare. Sonography findings of myxoid fibroadenomas have been shown to be well-circumscribed or complex cystic masses, without calcification (5). The myxoid fibroadenomas in case 3 were well-circumscribed solid masses with some cystic portions on sonography. The bilaterality and multiplicity of the myxoid fibroadenomas in case 3 were similar with findings previously reported (5).
Overactivity of endocrine organs is another clinical manifestation in the Carney complex. The following types of endocrine gland tumors have been reported in the Carney complex: growth hormone-secreting pituitary adenomas, thyroid adenomas or carcinomas, and testicular tumors. Primary pigmented nodular adrenocortical disease (PPNAD) is another cause of endocrine overactivity.
Because the growth hormone level may be increased in patients with pituitary adenomas, acromegaly can occur (6). In the cases described herein, the growth hormone levels were increased; and pituitary adenomas were depicted on the brain MR images. Among our cases, one had gigantism (case 1).
The typical testicular tumor in the Carney complex is a large-cell calcifying Sertoli and Leydig cell tumor. The sonography findings show a bizarre pattern of dense clumps. Because large-cell calcifying Sertoli and Leydig cell tumors are more likely to be bilateral, therapeutic planning can be a dilemma. A bilateral orchiectomy provides excellent local control of the tumors, but infertility and poor quality of life result. More importantly, these tumors are nearly always benign and rarely associated with aromatization (7). In one report, patients with bilateral large-cell calcifying Sertoli and Leydig cell tumors related to the Carney complex, were treated with antiestrogen drugs and conservative methods rather than a bilateral orchiectomy (8). Because cases 1 and 2 did not present with clinical symptoms related to the testicular tumors, and there was no definite evidence of malignant tumors, we planned a conservative management strategy with regular follow-ups.
PPNAD, which occurs in about one-fifth of patients with the Carney complex, results in ACTH-independent Cushing's syndrome (9). The pathologic features of the adrenal lesions are as follows; 1) decreased, normal, or slightly increased total weight; 2) external and cut surfaces by small (less than 4 mm) black, brown, dark-green, red, or yellow nodules; and 3) cortical atrophy and disorganization of the normal zonation between the nodules. In case 2, although there was ACTH-independent Cushing's syndrome based on the laboratory data and enlargement of both adrenal glands with small nodules, the presence of large functional adrenal masses was enigmatic, because some nodules among the adrenal lesions in PPNAD can be more than 3 cm in diameter (10). We concluded that Cushing's syndrome, found in case 2 resulted from PPNAD. Consequently, the treatment of choice, bilateral adrenalectomy did not work.
In conclusion, the Carney complex is composed of multiple disease entities, and the clinical manifestations vary between patients. Herein, we have presented the typical and various clinical manifestations of the Carney complex in three patients. Because cardiac myxomas and malignant tumors increase the rate of mortality and morbidity, early diagnosis and proper management is essential for a better prognosis.
The recognition of the Carney complex and the accurate comprehension of its various clinical manifestations are important to diagnose and manage affected patients, and to screen their first-degree relatives.
Figures and Tables
Fig. 1
Case 1.
A, B. Cerebral infarction due to a cardiogenic embolism (case 1).
Diffusion weighted MR images show multiple high signal intensities involving the cerebellar hemispheres, pons, midbrain, thalami, left hippocampus, the body and splenium of the corpus callosum, both cerebral cortices, and subcortical white matter. These findings are typical of a cardiogenic embolic infarction.
C. A Pelvic CT scan shows the asymmetric enlargement and internal calcification in the left testis.
D. A scrotal sonography shows complex masses with irregular calcification patterns and heavy posterior acoustic shadowing.
Increased arterial flow around the nodules is also noted.
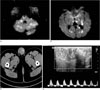
Fig. 2
Case 2.
A. Coronal reformatted CT images with contrast enhancement show the lobular mass (5 × 4 × 6 cm) with mild contrast enhancement in the left atrium.
B. Cardiac myxoma shows tumor cells (arrow) concentrated just beneath the surface, and surrounded by an abundant myxoid stroma (a) (hematoxylin and eosin stain, original magnification × 1.0).
C. Arterial CT image shows a large mass (arrow, 3.5 cm in maximum diameter) with strong enhancement in the left adrenal gland.
D. An axial CT scan after 10 minutes shows the delayed wash-out of the contrast agent in the nodule (open arrow). The absolute wash-out percentage was calculated to be 63%.
E, F. Coronal (E) and sagittal (F) MR images with gadolinum-enhancement show an enlargement of the pituitary gland with multiple enhancing nodules.
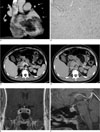
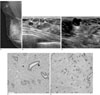
Fig. 3
Myxoid tumors of the breast and axilla in case 3.
A. A mammography, in which the right MLO view, shows a well-circumscribed round nodule with a slightly lobular margin in the right axilla and two nodular asymmetries in the right breast.
B. A breast sonography shows variable-sized, multiple ovoid nodules (arrows) with well-circumscribed margin and slightly heterogeneous echogenicity in both breasts.
C. An ultrasound-guided biopsy on the right axilla shows a lobular complex cystic mass (2 × 1 × 1 cm) with posterior acoustic enhancement (open arrow).
D. A photomicrograph, obtained from the right breast mass, shows attenuated ductal structures (arrowhead) among the myxoid stroma (a), suggesting a fibroadenoma with abundant myxoid stromal change (hematoxylin and eosin stain, original magnification × 1.0).
E. A photomicrograph, obtained from right axillary mass, shows that the tumor is hypocellular and consists of cytologically benign, widely scattered cells with an ill-defined cytoplasm suspended in the myxoid stromal matrix. These findings are consistent with myxoma (hematoxylin and eosin stain, original magnification × 4.0).
References
1. Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore). 1985; 64:270–283.
2. Carney JA, Hruska LS, Beauchamp GD, Gordon H. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Mayo Clin Proc. 1986; 61:165–172.
3. Radin R, Kempf RA. Carney complex: Report of three cases. Radiology. 1995; 196:383–386.
4. Carney JA. Differences between nonfamilial and familial cardiac myxoma. Am J Surg Pathol. 1985; 9:53–55.
5. Courcoutsakis NA, Chow CK, Shawker TH, Carney JA, Stratakis CA. Syndrome of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas (Carney complex): breast imaging findings. Radiology. 1997; 205:221–227.
6. Boikos SA, Stratakis CA. Pituitary pathology in patients with Carney complex: growth-hormone producing hyperplasia or tumors and their association with other abnormalities. Pituitary. 2006; 9:203–209.
7. Washecka R, Dresner MI, Honda SA. Testicular tumors in Carney's complex. J Urol. 2002; 167:1299–1302.
8. Brown B, Ram A, Clayton P, Humphrey G. Conservative management of bilateral sertoli cell tumors of the testicle in association with the Carney complex: a case report. J Pediat Surg. 2007; 42:E13–E15.
9. Carney JA. Carney complex: the complex of myxomas, spotty pigmentation, endocrine overactivity, and schwannomas. Semin Dermatol. 1995; 14:90–98.
10. Sarlis NJ, Chrousos G, Doppman J, Carney J, Stratakis C. Primary pigmented nodular adrenocortical disease: reevaluation of a patient with Carney complex 27 years after unilateral adrenalectomy. J Clin Endocrinol Metab. 1997; 82:1274–1278.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download