Abstract
A pilomyxoid astrocytoma (PMA) is a recently identified low-grade neoplasm that was previously classified as a pilocytic astrocytoma (PA), yet demonstrates unique histological features and more aggressive behavior. Although a PMA is generally a tumor of early childhood and typically occurs in the hypothalamic/chiasmatic region, it can mimic cortical tumors, especially in adults. We report the MR findings of a PMA presenting as a cortical brain tumor in an adult with neurofibromatosis 1 (NF1).
A pilomyxoid astrocytoma (PMA) is a recently classified, low-grade neoplasm of early childhood or adolescence which typically occurs in the hypothalamic/chiasmatic region (1). Only rarely has this tumor been reported in adults. We report the MR findings of a pilomyxoid astrocytoma presenting as a cortical brain tumor in an adult with neurofibromatosis 1 (NF1), as well as a review of the pertinent literature.
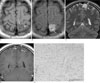
A 22-year-old woman with neurofibromatosis 1 (NF1) was admitted to our institution with a generalized seizure. She had no focal neurologic deficit. On MR imaging, a well-defined, solid mass (2 × 2 cm) was noted in the left medial parietal lobe and showed slightly low-signal intensity and homogeneous high-signal intensity relative to the surrounding grey matter on T1-weighted and T2-weighted MR images, respectively (Figs. 1A, B). Coronal T2-weighted MR images revealed that the mass was primarily located in the cortex of the parietal lobe (Fig. 1C). After gadolinium administration, no significant enhancement was observed within the mass (Fig. 1D), nor was there any evidence of perilesional edema or midline shift. Complete tumor removal was performed. The microscopic findings indicated that the pilomyxoid astrocytoma consisted of a monomorphic population of piloid cells in a loose fibrillary and myxoid background without the presence of Rosenthal fibers (Fig. 1E). The PMA tumor cells had round to oval nuclei and long thin cytoplasmic processes that stained positively with antibodies for GFAP. No mitotic figures or necrosis w seen and immunohistochemical stains for the proliferation marker MIB-1 showed a labeling index of less than 1%. Immunohistochemical stains for synaptophysin were strongly positive.
A pilomyxoid astrocytoma (PMA) is a recently classified WHO grade II neoplasm that was formerly described as a variant of a pilocytic astrocytoma (PA) with a less favorable prognosis (1). PMAs have unique histological features including a monomorphic pattern of elongated piloid cells in a loose fibrillary and myxoid background and without the presence of Rosenthal fibers which are characteristic of PAs (2). In addition, PMAs lack a biphasic pattern, protoplasmic cells, and eosinophilic granular bodies. PMAs have been reported to show more aggressive behavior, with a higher local recurrence than the typical PA and also showed a tendency to spread throughout the neuraxis (3). The PMA is generally a tumor of early childhood or adolescence and typically occurs in the hypothalamic/chiasmatic region, which is also often affected by classical pilocytic astrocytomas (1). However, in a recent study, PMAs were illustrated in a wide age range and location within the body (4). In our study, we add a PMA case that mimics a cortical brain tumor in an adult with neurofibromatosis 1 (NF1).
Pilomyxoid astrocytomas in adults may not necessarily be located in the neuroaxis such as the optic chiasm, hypothalamus, and the 3rd ventricle. Usually, pilomyxoid astrocytomas which develop in suprasellar and chiasmatic lesions appear as a well-enhanced mass with a cystic portion, similar to a pilocytic astrocytoma. The most distinctive radiologic features suggesting a PMA rather than a typical PA is a predominantly solid tumor, homogeneous enhancement, and relatively frequent intratumoral hemorrhage (45). In our case, the PMA presented with T2 high signal intensity of the solid cortical mass without intratumoral hemorrhage or significant enhancement after gadolinium administration, while simultaneously taking into consideration its usual appearance as a low-grade, cortical tumor (i.e., dysembryoblastic epithelial tumor (DNET) or ganglioglioma) as usually identified on the differential diagnosis. Although PMAs have been known to typically occur in the chiasmatic-hypothalamic region in young children, a few recent studies have suggested that PMAs can present in the hemispheres or cortex of the brain in adults rather than in the neuroaxis as seen in children (467). As a result, when a mass is identified in the cerebral hemisphere of an adult, correctly diagnosing a PMA is a difficult task based on MR imaging results.
Although the relationship of PMA to NF 1 is unclear, the incidence of PMA in the context of NF1 might be seen in the same perspective as PAs, which are frequently detected in NF1. As the alteration of the NF1 gene can lead to a large variety of complications including the development of tumors, intracranial tumors account for the largest group of NF1-associated neoplasms primarily affecting children under the age of 10 years (8). The most common CNS tumor in the NF1 population is the optic pathway glioma and the majority of these tumors are PAs (89). As a variation of the PAs, PMAs could be an intracranial neoplasm associated with NF1. There have been three reported intracranial PMA cases associated with NF 1 in the literature (Table 1) (4910). All of these lesions were located in the neuroaxis; the optic pathway, hypothalamus, an third ventricle region. Our case is the only adult case reported to have NF1 and a developed PMA in the cortex. We speculated that the cortical location of the tumor could play a role in adult cases of this tumor, which is typically found in early childhood.
In summary, the PMA is a rare variant of the PA, which is generally a tumor of early childhood and typically occurs in the hypothalamic and chiasmatic region. However, PMAs could appear as a cortical tumor in adults and may have similar MR features to those of other cortical tumors.
Figures and Tables
Fig. 1
A pilomyxoid astrocytoma in a 22-year-old woman presenting with a seizure.
A. An axial, T1-weighted MR image shows a well-circumscribed, low-signal intensity solid mass in the cortex of the left parietal lobe (arrows).
B. On FLAIR images, the mass is shown as high-signal intensity without hemorrhage or peritumoral edema. The cyst-like portion at the center of the mass is caused by the partial volume of the overlying sulcus of the parietal lobe (arrow).
C. The mass appears to be located mainly in the cortex of the parietal lobe on the coronal, T2-weighted MR images (arrows).
D. After gadolinium administration, there is no significant enhancement within the mass (arrows).
E. A photomicrograph (original magnification, ×100; hematoxylin-eosin stain) shows that the tumor is composed of a monomorphic population of cells in a myxoid background. The tumor cells have round to oval nuclei and a spindle cytoplasmic process. There are no Rosenthal fibers or eosinophilic granular bodies. Also, no mitotic figure is seen.
References
1. Brat DJ, Parisi JE, Kleinschmidt-DeMasters BK, Yachnis AT, Montine TJ, Boyer PJ, et al. Surgical neuropathology update: a review of changes introduced by the WHO classification of tumours of the central nervous system, 4th edition. Arch Pathol Lab Med. 2008; 132:993–1007.
2. Tihan T, Fisher PG, Kepner JL, Godfraind C, McComb RD, Goldthwaite PT, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999; 58:1061–1068.
3. Komotar RJ, Burger PC, Carson BS, Brem H, Olivi A, Goldthwaite PT, et al. Pilocytic and pilomyxoid hypothalamic/chiasmatic astrocytomas. Neurosurgery. 2004; 54:72–79.
4. Linscott LL, Osborn AG, Blaser S, Castillo M, Hewlett RH, Wieselthaler N, et al. Pilomyxoid Astrocytoma: expanding the Imaging Spectrum. AJNR Am J Neuroradiol. 2008; 29:1861–1866.
5. Arslanoglu A, Cirak B, Horska A, Okoh J, Tihan T, Aronson L, et al. MR imaging characteristics of pilomyxoid astrocytomas. AJNR Am J Neuroradiol. 2003; 24:1906–1908.
6. Komotar RJ, Mocco J, Zacharia BE, Wilson DA, Kim PY, Canoll PD, et al. Astrocytoma with pilomyxoid features presenting in an adult. Neuropathology. 2006; 26:89–93.
7. Gottfried ON, Fults DW, Townsend JJ, Couldwell WT. Spontaneous hemorrhage associated with a pilomyxoid astrocytoma: case report. J Neurosurg. 2003; 99:416–420.
8. Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994; 125:63–66.
9. Rodriguez FJ, Perry A, Gutmann DH, O'Neill BP, Leonard J, Bryant S, et al. Gliomas in neurofibromatosis type 1: a clinicopathologic study of 100 patients. J Neuropathol Exp Neurol. 2008; 67:240–249.
10. Khanani MF, Hawkins C, Shroff M, Dirks P, Capra M, Burger PC, et al. Pilomyxoid astrocytoma in a patient with neurofibromatosis. Pediatr Blood Cancer. 2006; 46:377–380.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download