Abstract
Purpose
To evaluate high cortical signal intensity and chronologic changes for diffusion-weighted MR imaging (DWI) in sporadic Creutzfeldt-Jakob disease.
Materials and Methods
We retrospectively analyzed the DWI results of 16 patients with probable CJD (according to WHO criteria) and evaluated the distribution, extent and bilaterality of the lesions in the cortex, basal ganglia and thalamus. We also reviewed the chronologic changes of the lesions by evaluating the follow-up MR examination results in 8 of 16 patients.
Results
Cortical abnormalities were present in 15 (94%) of 16 patients. Isolated cortical involvement was present in 6 patients (40%), while the combined involvement of the cortex and basal ganglia was present in 9 patients (60%). The distribution of the lesions was bilateral in 12 patients and predominantly on the right side in 8 patients. Upon follow-up MR imaging, the cortical lesions showed progress in terms of extent and signal intensity. Basal ganglia abnormalities were present in 9 of 15 patients. Moreover, 4 of 6 patients who had no abnormal signal intensity in the basal ganglia on the initial MR imaging results, showed abnormally high signal intensity upon follow-up MR imaging.
Figures and Tables
Fig. 1
Isolated cortical high signal intensity lesions in CJD.
A, B. Axial diffusion-weighted images of a 67-year-old woman show abnormal high signal intensities in bilateral parietal and occipital lobes, and right insula (arrows).
C, D. Axial diffusion-weighted images of a 41-year-old man show abnormal high signal intensities in bilateral frontal and parietal lobes (arrows).
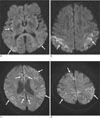
Fig. 2
Combined cortex and basal ganglia high signal intensities in CJD.
A, B. Axial diffusion-weighted images of a 53-year-old man show abnormal high signal intensities in right striatum, right frontal lobe, left medial frontal lobe, and right medial parietal lobe (arrows).
C. Diffusion-weighted image of a 78-year-old woman shows cortical high signal intensities in right cerebral hemisphere, and focally left frontal and occipital lobes (arrows).
D. FLAIR image of the same patient shows subtle hyperintensity of the lesions (arrows).
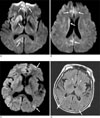
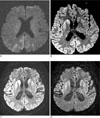
Fig. 3
The chronologic changes of a 59-year-old man with CJD. Diffusion-weighted images were obtained at 30 days (A), 80 days (B), 95 days (C), and 165 days after onset of symptoms.
A. Diffusion-weighted image shows cortical high signal intensities in bilateral parietal lobes.
B. Diffusion-weighted image shows high signal intensities in bilateral cerebral hemisphere and bilateral striatum. The signal intensity of the lesions is more prominent in right than left cerebral hemisphere.
C. Diffusion-weighted image shows more increased extent and signal intensity of the high signal intensity lesions in left cerebral hemisphere and striatum.
D. The last follow-up diffusion-weighted image shows more decreased high signal intensities in the cerebral cortex and striatum, and more progressed cerebral atrophy than C.
References
1. Johnson RT, Gibbs CJ Jr. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998; 339:1994–2004.
2. Ukisu R, Kushihashi T, Tanaka E, Baba M, Usui N, Fujisawa H, et al. Diffusion-weighted MR imaging of early-stage Creutzfeldt-Jakob disease: typical and atypical manifestations. Radiographics. 2006; 26:Suppl 1. S191–S204.
3. Mao-Draayer Y, Braff SP, Nagle KJ, Pendlebury W, Penar PL, Shapiro RE. Emerging patterns of diffusion-weighted MR imaging in Creutzfeldt-Jakob disease: case report and review of the literature. AJNR Am J Neuroradiol. 2002; 23:550–556.
4. Gertz HJ, Henkes H, Cervos-Navarro J. Creutzfeldt-Jakob disease: correlation of MRI and neuropathologic findings. Neurology. 1988; 38:1481–1482.
5. Meissner B, Kortner K, Bartl M, Jastrow U, Mollenhauer B, Schroter A, et al. Sporadic Creutzfeldt-Jakob disease: magnetic resonance imaging and clinical findings. Neurology. 2004; 63:450–456.
6. Tschampa HJ, Kallenberg K, Kretzschmar HA, Meissner B, Knauth M, Urbach H, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007; 28:1114–1118.
7. Meissner B, Kallenberg K, Sanchez-Juan P, Krasnianski A, Heinemann U, Varges D, et al. Isolated cortical signal increase on MR imaging as a frequent lesion pattern in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2008; 29:1519–1524.
8. Ukisu R, Kushihashi T, Kitanosono T, Fujisawa H, Takenaka H, Ohgiya Y, et al. Serial diffusion-weighted MRI of Creutzfeldt-Jakob disease. AJR Am J Roentgenol. 2005; 184:560–566.
9. Young GS, Geschwind MD, Fischbein NJ, Martindale JL, Henry RG, Liu S, et al. Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: high sensitivity and specificity for diagnosis. AJNR Am J Neuroradiol. 2005; 26:1551–1562.
10. Brown P, Cathala F, Castaigne P, Gajdusek DC. Creutzfeldt-Jakob disease: clinical analysis of a consecutive series of 230 neuropathologically verified cases. Ann Neurol. 1986; 20:597–602.
11. Kim HC, Chang KH, Song IC, Lee SH, Kwon BJ, Han MH, et al. Diffusion-weighted MR imaging in biopsy-proven Creutzfeldt-Jakob disease. Korean J Radiol. 2001; 2:192–196.
12. Bahn MM, Parchi P. Abnormal diffusion-weighted magnetic resonance images in Creutzfeldt-Jakob disease. Arch Neurol. 1999; 56:577–583.
13. Mittal S, Farmer P, Kalina P, Kingsley PB, Halperin J. Correlation of diffusion-weighted magnetic resonance imaging with neuropathology in Creutzfeldt-Jakob disease. Arch Neurol. 2002; 59:128–134.
14. Murata T, Shiga Y, Higano S, Takahashi S, Mugikura S. Conspicuity and evolution of lesions in Creutzfeldt-Jakob disease at diffusion-weighted imaging. AJNR Am J Neuroradiol. 2002; 23:1164–1172.
15. Gray H. Gray's anatomy. 35th ed. London, England: Longman;1973. p. 976–979.
16. Masters CL, Harris JO, Gajdusek DC, Gibbs CJ Jr, Bernoulli C, Asher DM. Creutzfeldt-Jakob disease: patterns of worldwide occurrence and the significance of familial and sporadic clustering. Ann Neurol. 1979; 5:177–188.
17. Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol. 1996; 53:913–920.
18. Wakayama Y, Shibuya S, Kawase J, Sagawa F, Hashizume Y. High neuron-specific enolase level of cerebrospinal fluid in the early stage of Creutzfeldt-Jakob disease. Klin Wochenschr. 1987; 65:798–801.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download