Abstract
A malignant fibrous histiocytoma (MFH) is the most common soft tissue sarcoma encountered during adulthood, but the breast is not a common site of involvement for MFH. Several investigators have reported the histopathological and biological features of a MFH involving the breast, but only a few reports have focused on the imaging findings of breast MFHs. To emphasize the importance of arriving at a preoperative diagnosis for the treatment implications, we report here the imaging findings, including the mammography, US and MRI findings, for a MFH of the breast of a 53-year-old woman who presented with a rapid growing huge mass in the right breast.
A malignant fibrous histiocytoma (MFH) most frequently develops in the deep fascia or skeletal muscle of the limbs, retroperitoneum and abdominal cavity (123). Primary breast MFH is extremely rare, and a few such cases have been reported that have described the imaging findings of breast MFH (345).
We report the imaging findings of primary breast MFH in a 53-year-old woman, including the mammography, US and MRI. To the best of our knowledge, this is the first case report with the MR imaging features of MFH of the breast.
A 53-year-old woman was admitted to our hospital due to fever and a rapidly growing huge tender mass in her right breast. Eleven months prior to visiting our hospital, the patient had undergone a vacuum-assisted breast biopsy (VABB) at a local clinic for an incidentally detected mass during screening breast US (Fig. 1A), and no abnormality was seen on mammography. The histology findings of the VABB were no definite malignancy, and the findings suggested a fibroadenomatous change. Three months after the VABB, follow-up US revealed a 1cm sized irregular anechoic lesion (Fig. 1B). Aspiration was done for the palpable lump at the biopsy-site one month afterward and brownish fluid was noted, so this was considered as a slow resolving post-biopsy hematoma or recurrent fluid collection. But on a retrospectively review, the mass was considered to be residual tumor, and after 5 months, the mass started growing again (Fig. 1C). Four months after this, the patient was transferred to our hospital via the emergency room due to progressive growth of the lesion and the patient had had a high fever that had lasted for three days. The mass was firm and extremely large, and mammography could not be performed. An US exam done in our hospital revealed a well-defined huge heterogeneous mixed echoic mass with variable sized cystic areas (Fig. 1D) and high vascularity was seen in the solid portion on power Doppler US (Fig. 1E). We assessed the lesion to be BI-RADS category 5 and an US-guided core biopsy was performed. The histopathology revealed an unclassified malignant tumor and the differential diagnosis included malignant myoepithelial tumor, sarcomatoid carcinoma or sarcoma. Pre-operative breast MRI was performed with a 1.5-T MR imaging system (Intera, Ver. 11.1.4.3; Philips, Best, the Netherlands). On the MR images, a 12×6×16 cm sized, smoothly well-defined multi-lobulated mass had replaced almost the entire right breast. The mass showed intermediate signal intensity on the T1-weighted images and central, variable, mixed heterogeneous (from very high to very low) signal intensity and peripheral intermediate signal intensity on the T2-weighted images (Figs. 2A, B). A gadolinium-enhanced dynamic scan showed the early rapid peripheral irregular thick rim enhancement of the mass (Fig. 2C).
The patient underwent a simple mastectomy and ipsilateral axillary lymph nodes dissection. On the macroscopic examination, the breast was mostly replaced by the tumor mass; the mass had a relatively well-delineated and lobulated margin and the tumor measured 16 × 12 × 6 cm. The cut surface was grayish yellow with a "fish flesh" appearance, and necrosis and hemorrhage were noted (Fig. 3A). The histopathological findings showed diffuse proliferations of atypical spindle cells with a whorling pattern (Fig. 3B). The tmor cells exhibited round to oval vesicular nuclei with plump eosinophilic or foamy granular cytoplasm (Fig. 3C). A diffuse positive reaction was seen on CD10 immunostaining. The expression of other epithelial markers (MOC31, CK7 and CK20), HMB-45, c-Kit, estrogen, progesterone receptors and Her-2 were negative. The staining for p53 was positive. According to the histological and immunohistochemical staining results, this case was finally diagnosis as a malignant tumor, and especially a high-grade malignant fibrous histiocytoma after ruling out other malignancies. We retrospectively reviewed the vacuum-assisted breast biopsy specimen, and there was only loose connective tissue, suggesting neither definite malignancy nor fibroadenoma.
Primary malignancies of the breast stromal elements and sarcomas of a mesenchymal origin account for less than 1% of all breast neoplasm. To the best of our knowledge, 28 cases of a breast primary MFH have been reported in the English medical literature.
Secondary MFH of the breast can be derived from malignant transformation according to several predisposing factor, including radiation therapy, surgery, a burn scar and other malignancy such as a malignant phyllodes tumor. Almost all the cases of secondary MFH of the breast have developed after receiving radiation therapy for breast cancer treatment, and usually within 10 to 30 years after a long latent period (126).
In our case, the patient acknowledged the presence of a palpable mass four months after receiving a VABB, which is too short a time to have developed a secondary MFH. Considering that the patient did not have a history of undergoing radiation therapy, which is the most frequent reported predisposing factor, we think that the disease in this case is a primary breast MFH and the VABB was the VABB was performed during an intervening episode of disease progression or the biopsy missed the tumor tissue. Generally, soft-tissue MFHs manifest as a painless, progressive growing palpable mass over several months and they are sometimes accompanied with a large hematoma or intratumoral hemorrhage (12), like what occurred in our case. Fever is not a common symptom of patients with MFH, but our patient had fever that was probably due to the extensive tumoral necrosis.
Histologically, MFHs are divided into five subtypes that include storiform-pleomorphic, myxoid, giant cell, inflammatory and angiomatoid (1235). Primary and secondary soft-tissue MFHs, including breast MFHs, have a poor prognosis without any relation to the subtypes. In our case, the subtype could not be classified as the diagnosis was made after all of the other beast sarcomas were ruled out according to the negative expression of specific markers and the lack of a prominent cellular type.
According to the previous reports of the imaging findings of breast MFHs and MFHs at other sites (12457), US depicts a well-defined heterogeneous solid mass and the hypoechoic areas representing necrosis. The CT images reveal a well-defined large lobulated mass with iso- or hypodense areas that correspond to myxoid lesions or necrosis. Tumors may have areas of high attenuation hemorrhage. The solid component is enhanced after intravenous contrast injection. MR imaging of general MFHs shows low to intermediate signal intensity on the T1-weighted images, heterogenous high signal intensity on the T2-weighted images and enhancement of the solid component on the Gd-enhanced MR images. Extensive areas of hemorrhage can obscure an underlying neoplasm. We performed MRI instead of CT, and MRI showed findings that were similar to those of the above mentioned general MFHs.
A primary breast MFH is rare malignant neoplasm, but one should consider the possibility of this tumor when a patient presents with a progressive growing breast mass with variable solid and cystic foci of hemorrhage and necrosis.
Figures and Tables
Fig. 1
The ultrasound exam that was done before the VABB.
A. There is a 1.87 cm × 1.12 cm sized, irregular, parallel, microlobulated hypoechoic mass in the 10 o'clock direction of the right breast (arrows).
B. On the ultrasound exam that was done 3 months after the VABB, there is a 1 cm sized irregular anechoic lesion at the biopsy site (arrows).
C. On the ultrasound exam performed 9 months after the VABB, there is a 3.5 × 2.8 × 3 cm sized round, circumscribed mixed echoic mass with posterior enhancement and a partial cystic component at the biopsy site of the right breast (arrows).
D. The ultrasound exam performed 4 months afterward reveals a well-defined, huge heterogenous echogenic mass with variable cystic areas that occupy almost the entire right breast.
E. The mass shows high vascularity in the solid portion on power Doppler US.
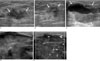
Fig. 2
MR images of the right breast.
A, B. There is a 12 cm sized, smoothly well-defined multilobulated intermediate signal intensity mass on the T1 weighted image (A) and the T2 weighted MR image (B). The mass shows central variable, mixed heterogeneous (from very high to very low) signal intensity and peripheral intermediate signal intensity.
C. The Gd-enhanced scan shows the early rapid irregular thick peripheral rim enhancement of the mass.

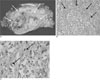
Fig. 3
On the gross specimen, the tumor mass has a relatively well-delineated and lobulating margin, and the mass measures 16×12×6 cm.
A. The cut surface is grayish yellow with a "fish flesh" appearance and necrosis and hemorrhage (arrows).
B. The histopathology findings of the breast MFH (H & E stain, ×200, ×400) show a diffuse proliferation of atypical spindle cells with a whorling pattern (arrows).
C. The tumor cells exhibit round to oval vesicular nuclei with plump eosinophilic or foamy granular cytoplasm (arrows).
References
1. Murphey MD, Gross TM, Rosenthal HG. From the archives of the AFIP. Musculoskeletal malignant fibrous histiocytoma: radiologicpathologic correlation. Radiographics. 1994; 14:807–826.
2. Son E, Park J, Jeon H, Cho S. Malignant fibrous histiocytoma (MFH) in axilla. Yonsei Med J. 2004; 45:736–738.
3. Oh SJ, Kim KM, Hong TH, Park WC, Kim JS, Jung SS. Giant cell malignant fibrous histiocytoma of the breast: a case report. J Korean Med Sci. 2004; 19:477–480.
4. Hocevar M, Marinsek ZP, Zidar A. Myxofibrosarcoma of the breast as an unusual variant of malignant fibrous histiocytoma: report of a case. Surg Today. 2004; 34:752–754.
5. Kijima Y, Umekita Y, Yoshinaka H, Taguchi S, Owaki T, Funasako Y, et al. Stromal sarcoma with features of giant cell malignant fibrous histiocytoma. Breast Cancer. 2007; 14:239–244.
6. Ugurlu K, Turgut G, Kabukcuoglu F, Ozcan H, Sanus Z, Bas L. Malignant fibrous histiocytoma developing in a burn scar. Burns. 1999; 25:764–767.
7. Ajisaka H, Maeda K, Uchiyama A, Miwa A. Myxoid malignant fibrous histiocytoma of the breast: report of a case. Surg Today. 2002; 32:887–890.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download