Abstract
Purpose
To evaluate imaging findings of cystic hypersecretory carcinoma (CHC) of the breast, with an emphasis of the sonographic (US) features, and to correlate the US findings with the histology.
Materials and Methods
During the last 13 years, six women with a mean age of 43 years were histologically confirmed with CHC of the breast. We retrospectively reviewed the clinical records, US images, and correlated them with histological findings.
Results
US showed a large complex cystic mass lined by a thin wall (n = 2), multiple small cysts in focally heterogeneous background parenchyma without predominant mass (n = 3), and an irregular mass with an ill-defined margin and ductectasia (n = 1). The pathology revealed that the thin walls of the cysts were all cystic hypersecretory intraductal carcinomas, while the solid portions of the lesions varied from benign intraductal papillomas to minimal infiltrating ductal carcinoma in the background of the cystic hypersecretory intraductal carcinomas.
Cystic hypersecretory duct carcinoma (CHC) of the breast is a rare subtype of ductal carcinoma. The characteristic histological features of CHC include cystic dilatation of the ducts and acini filled with thyroid colloid-like eosinophilic material. The epithelium lining the cysts produces foci of micropapillary carcinoma (123). CHC of the breast behaves in a low-grade fashion but can evolve into high-grade carcinoma with distant metastasis (1234567). The correct diagnosis and appropriate treatment is therefore important. To our knowledge, there have been only two reports of the imaging findings of cystic hypersecretory duct carcinoma of the breast (89). Thus, the purposes of our study were to evaluate the imaging characteristics of CHC of the breast, emphasizing the sonographic (US) features, and to correlate the US findings with the histology.
Institutional Review Board approval and informed consent were not required for this retrospective study. From July 1995 to December 2008, six women at our hospital were histologically confirmed with CHC of the breast. All patients underwent preoperative imaging with a mammography, US, or MR imaging.
In all patients, CHC was confirmed after breast surgery. Four patients received a modified radical mastectomy, while two patients underwent breast conservation surgery with sentinel node biopsy. A preoperative biopsy was performed in all patients using 14G true cut needles (n=5) under US guidance or surgical excision (n=1). All patients were women aged between 33 and 52 years old (mean age 43 years).
We retrospectively analyzed the US features of the tumors and correlated those features with the histologic findings. Clinical data, including clinical symptoms, tumor stage, and tumor recurrence or survival, were collected through a review of the clinical records.
Breast US were performed for all tumors before surgery or biopsy using either the HDI5000 (Advanced Technology Laboratories, Bothell, WA, USA) or the LOGIQ700 ultrasound scanner (GE Medical Systems, Milwaukee, WI, USA) equipped with 12-5-MHz lineararray transducer. All sonographic examinations were performed or supervised by three experienced breast radiologists. We retrospectively analyzed the imaging characteristics of the tumors, which include the size, shape, margin, internal echogenicity, vascularity of the solid portion, and BI-RADS category.
A preoperative core biopsy was performed with at least four needle passes at the different sites of the lesions as well as targeting the solid portion within the lesion.
Results of the preoperative percutaneous biopsy were re-evaluated for correlation with the US findings of the breast masses, and were compared with the final histological results after surgery. Hematoxylin-eosin-stained slides from the surgical specimens of five tumors were re-evaluated to confirm the morphologic diagnosis and histologic grading, as well as correlating the results with US features. The pathologic slide of one tumor was not available; therefore, the previous pathologic report was evaluated. Histologic subtyping or grading, generally accepted as a guide to prognostic evaluation, was documented for each tumor.
Five of six tumors manifested as palpable masses (Table 1). Two of the palpable masses were painful and the remaining three palpable masses also had bloody nipple discharge. The sixth tumor that was not diagnosed as a palpable mass was asymptomatic and detected on the screening mammogram. The mean tumor size was 6.6 cm (range, 2.8 - 12 cm). No cases had a metastatic axillary lymph node or distant metastasis at the time of diagnosis. The TNM stages of the tumors were TisN0M0 in five cases and T1N0M0 in one case. The clinical and imaging follow-up (mean: 7 years, range: 2 - 13 years) of the five patients revealed no tumor recurrence after treatment.
In two cases, US showed complex cystic masses lined by a thin wall with 7.0 cm and 2.8 cm diameters, respectively (Fig. 1), while three cases had multiple small cysts in focally heterogeneous background parenchyma without a predominant mass (Fig. 2). One case showed an irregular mass with an ill-defined margin and ductal dilatations. Solid portions were isoechoic in all cases, and most cases (n=5) showed increased vascularity, except for one case that had no Color Doppler study. The US findings of each tumor are summarized in Table 2.
In each patient, mammogram results were also available: Three of the tumors showed hyper-dense masses, whereas two showed focal asymmetry, and the remaining tumor showed no abnormal density on the mammogram. In addition, amorphous calcifications associated with the lesions were noted in three cases, including one with no abnormal density.
MR imaging was performed for one lesion (Fig. 2). The lesion consisted of multiple small cysts which showed hyperintensity on a T2-weighted image and hypointensity on a T1-weighted image. In addition, segmental enhancement was noted with multifocal washout foci on the dynamic study.
The histological results of the biopsy and surgery for all the tumors are summarized in Table 3. An US-guided core biopsy was performed in five patients, whereas an excision biopsy was performed preoperatively in one patient. The histological diagnoses after the core biopsy were intraductal papillomas in three cases, intraductal papillary carcinoma in one case, and atypical ductal hyperplasia (ADH) in one case. One patient was diagnosed with CHC after an excision biopsy.
All patients underwent surgery. Three patients who were diagnosed with intraductal papillomas from a core needle biopsy also underwent additional surgery for the discordant result from the image-pathology (n=1) and for eliminating bloody nipple discharge (n=2). A modified radical mastectomy was performed in four patients, whereas breast conservation surgery was performed in two patients. One patient with multiple foci of minimal infiltrating ductal carcinoma in the background of cystic hypersecretory intraductal carcinoma (T1N0M0), was treated by a combination of chemotherapy and radiation therapy after a modified radical mastectomy. One patient with cystic hypersecretory intraductal carcinoma received radiation therapy after breast conservation surgery.
The mean tumor size was 6.6 cm (2.8 - 12 cm); however, five lesions were intraductal carcinomas and the only invasive carcinoma was in the T1 stage. The one invasive carcinoma was palpable, but the pathologic results of the large cystic portion revealed cystic hypersecregory intraductal carcinoma and rare multicentric microscopic foci of invasive carcinoma (approximately 0.9/0.5/0.3 cm).
The histologic examination revealed that the thin walls of the cysts, which were filled with secretions, were all cystic hypersecretory intraductal carcinomas, while the solid portions of the lesions varied from benign intraductal papillomas to invasive carcinomas.
A percutaneous core biopsy targeting the solid portion of the lesion showed false negative results in three cases, as opposed to a high-risk underestimation in one case. On the final pathology after surgery, three lesions showed cystic hypersecretory intraductal carcinomas within the solid portion, while the others only showed benign IDP in the solid portion.
CHC was first described by Rosen and Scott in 1984 as a distinctive rare variant of ductal carcinoma of the breast (1). Cystic hypersecretory lesions of the breast have a spectrum of morphologic features ranging from benign cystic hypersecretory hyperplasia to intermediate cystic hypersecretory hyperplasia with atypia and frankly malignant CHC (2). The clinical presentations of CHC are similar to those of other forms of ductal carcinoma in situ. Skalova et al. (10) reported five cases of CHC that showed a large palpable mass. The maximum diameter of the lesions ranged between 70 and 80 mm. The usual clinical presentation of CHC is a large, palpable abnormality with localized pain and, rarely, nipple discharge (11). However, the clinical manifestations of CHC are rather non-specific and do not allow for the distinction of this lesion from common fibrocystic changes. In our study, five lesions manifested as palpable masses. Furthermore, two of the five palpable masses were painful, whereas the remaining three also had bloody nipple discharge. One of the six tumors was asymptomatic and was detected with a routine mammography. The mean size of our cases was 6.6 cm, but most of them were detected in an early stage with palpable masses and large cystic portions.
According to the two previous studies of the radiologic features of CHC in three patients, two of the patients who underwent sonographic examination showed an irregular, ill-defined hypoechoic mass with internal hyperechoic foci and accompanying dilated ducts and, additionally, small cysts in one of the two patients (8). The third patient had multiple small aggregated, anechoic cysts with good through-transmission (9). In our cases, two patients had a large cystic mass lined by a thin wall, whereas three patients had poorly marginated non-mass lesions consisting of multiple cysts within a heterogeneous parenchyma. In two previously reported cases, the tumor sizes measured 4×3×2 cm and 2.5 cm while the mean tumor size in our cases was 6.2 cm.
CHC could be a diagnostic pitfall on a core needle biopsy, mainly because of sampling error. In our study, five patients who underwent a preoperative core needle biopsy had false negative results (n=3), or high-risk underestimation (n=1). Only one patient who underwent an excision biopsy had correct preoperative diagnosis.
Extensive sampling with correct targeting of the biopsy site, including not only the solid portion but also the wall of cysts, is critical for a correct diagnosis. This is because, in our cases, the solid portion between the cystic lesions can be benign or normal parenchyma, and that the tumor cells are present along the wall of the cyst.
Little is known about the clinical course of CHC. According to reports by Guerry et al. (2) and Herrmann et al (3), CHC seems to be a low-grade tumor that has indolent character during the in situ phase. However, when it has an invasive component, it behaves aggressively. While we frequently found cystic hypersecretory hyperplasia (CHH) in association with CHC (27), we had no case in which the progression of CHH to CHC could be documented.
In our study, although the lesions were palpable and the tumor size at the time of detection was large, no cases had lymph node metastasis or distant metastasis. Moreover, the histological grade was low in all cases. Our study had some weaknesses such a low number of patients with CHC, which was due to the rarity of this tumor in the breast. Longer follow-up and the study of additional cases will be necessary to further assess the imaging findings of CHC.
In conclusion, CHC can be detected early with a large palpable mass in a low pathologic stage as a result of predominant cyst formations. The characteristic US findings include a large complex cystic and solid mass or multiple small cysts in the background of focally heterogeneous parenchyma.
Figures and Tables
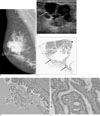 | Fig. 1A 52-year-old woman with cystic hypersecretory duct carcinoma of the right breast (Patient 6).A. Mediolateral oblique view of the mammography shows a hyperdense mass with lobulation, and without calcification in right upper outer breast. The mass shows relatively circumscribed, but a partially ill-defined margin in the posterior aspect.
B. US shows a complex cystic (arrow) and solid (arrowhead) mass with a thin cystic wall. The solid portion is isoechoic and has an ill-defined margin.
C. Histologically, the lesion consists of a few large cysts (arrow) and solid portions (arrowhead) (Hematoxylin-eosin stain, ×1).
D, E. The cystic wall (D) and solid portion (E) of the tumor. Disorderly proliferation of the lining epithelium suggests evidence of cystic hypersecretory intraductal carcinoma in the cyst wall. Benign intraductal papilloma was seen in the solid portion (Hematoxylin-eosin stain, ×200).
|
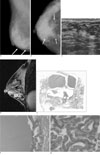 | Fig. 2A 46-year-old woman with cystic hypersecretory duct carcinoma of the right breast (Patient 4).A, B. Mediolateral oblique and craniocaudal views of the mammography show focal asymmetry with calcifications in right lower breast (arrows).
C. US shows a complex cystic appearance and the solid lesion consists of multiple small irregular cysts in a focally heterogeneous background parenchyma without a predominant mass.
D. A dynamic enhancement image of the lesion shows a segmental clumped nodular enhancement with multifocal small washout foci.
E. Histologically, the lesion had numerous cysts containing secretions, ranging from 0.1 cm to 2 cm in diameter. The cysts expelled a hemorrhagic brownish and slightly mucoid fluid. Occasionally, some cysts contained more solid soft white-grayish tissue (Hematoxylin-eosin stain, ×1).
F, G. Cystic wall (G) and solid portion (H) of the tumor. Both the wall of cysts and solid portion showed cystic hypersecretory intraductal carcinoma (Hematoxylin-eosin stain, ×200).
|
Table 3
Pathologic Results in Solid and Cystic Portion of the Lesions after a Preoperative Biopsy and Surgery

CNB: core needle biopsy
IDP: intraductal papilloma; IDPC: intraductal papillary carcinoma, ADH: atypical ductal hyperplasia
CHC: cystic hypersecretory intraductal carcinoma; IDPs: intraductal papillomatosis
Miniml IDC*: focus of minimal infiltrating ductal carcinoma in the background of cystic hypersecretory intraductal carcinoma
References
1. Rosen PP, Scott M. Cystic hypersecretory duct carcinoma of the breast. Am J Surg Pathol. 1984; 8:31–41.
2. Guerry P, Erlandson RA, Rosen PP. Cystic hypersecretory hyperplasia and cystic hypersecretory duct carcinoma of the breast. Pathology, therapy, and follow-up of 39 patients. Cancer. 1988; 61:1611–1620.
3. Herrmann ME, McClatchey KD, Siziopikou KP. Invasive cystic hypersecretory ductal carcinoma of breast: a case report and review of the literature. Arch Pathol Lab Med. 1999; 123:1108–1110.
4. Kim MK, Kwon GY, Gong GY. Fine needle aspiration cytology of cystic hypersecretory carcinoma of the breast. A case report. Acta Cytol. 1997; 41:892–896.
5. Lee WY, Cheng L, Chang TW. Diagnosing invasive cystic hypersecretory duct carcinoma of the breast with fine needle aspiration cytology. A case report. Acta Cytol. 1999; 43:273–276.
6. Lee JS, Lee YJ. Invasive cystic hypersecretory carcinoma of the breast: a case report. J Korean Med Sci. 2004; 19:149–151.
7. Shin SJ, Rosen PP. Carcinoma arising from preexisting pregnancy-like and cystic hypersecretory hyperplasia lesions of the breast: a clinicopathologic study of 9 patients. Am J Surg Pathol. 2004; 28:789–793.
8. Park JM, Seo MR. Cystic hypersecretory duct carcinoma of the breast: report of two cases. Clin Radiol. 2002; 57:312–315.
9. Park C, Jung JI, Lee AW, Cheon HM, Hahn ST, Lee JM. Sonographic findings in a patient with cystic hypersecretory duct carcinoma of the breast. J Clin Ultrasound. 2004; 32:29–32.
10. Skalova A, Ryska A, Kajo K, Di Palma S, Kinkor Z, Michal M. Cystic hypersecretory carcinoma: rare and poorly recognized variant of intraductal carcinoma of the breast. Report of five cases. Histopathology. 2005; 46:43–49.
11. Resetkova E, Padula A, Albarraccin CT, Sneige N. Pathologic quiz case: a large, ill-defined cystic breast mass. Invasive cystic hypersecretory duct carcinoma. Arch Pathol Lab Med. 2005; 129:e79–e80.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download