Abstract
Pancreatic arteriovenous malformation and isolated spontaneous dissection of the superior mesenteric artery are both rare maladies, and now they can be easily diagnosed due to the development of such noninvasive modalities as multi-detector computed tomography and magnetic resonance imaging. We report here on the multi-detector computed tomography and magnetic resonance imaging findings of a rare case of pancreatic arteriovenous malformation combined with isolated dissection of the superior mesenteric artery.
Pancreatic arteriovenous malformation (AVM) and isolated spontaneous dissection of the superior mesenteric artery (SMA) are both well-recognized entities, and these were first reported on by Halpern et al. in 1968 and Bauersfield in 1947, respectively (123). Noninvasive diagnostic modalities such as Doppler ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (MRI) all are known to provide adequate information for the confirmative diagnosis. Despite that Doppler US is sensitive for detecting the proximal portion of an SMA dissection and AVM, multiphased CT imaging have been revealed to be superior for determining the peripheral extent of a SMA dissection, and MR imaging is superior for determining the details of an AVM. In this report, we describe the MDCT and MRI findings of a unique case of a pancreatic AVM and an isolated SMA dissection that were discovered together in the same patient on his visit to the emergency department because of his epigastric pain.
A previously healthy 57-year-old man without any significant past medical history was admitted to the emergency department complaining of epigastric pain that radiated to the left flank. His initial blood pressure and body temperature were within the normal ranges, and physical examination revealed no tenderness in the abdomen. Laboratory tests of his blood and urine disclosed no abnormalities. CT scans of the abdomen and pelvis were obtained on a 16-MDCT scanner (SOMATOM Sensation 16, Siemens, Erlangen, Germany) both before and after intravenous administration of a contrast agent (including the arterial, portal venous and delay phases of contrast enhancement), and the sources images with a slice width of 1.25 mm and a slice interval of 1 mm were used for multi-planar reformation (MPR) by means of 3D viewing software. The CT obtained during the arterial phase disclosed an early-enhancing tangle of tortuous and dilated vasculature within the body of the pancreas, with feeding arteries from the transverse pancreatic artery and early-filling draining veins that led to the portal vein (Figs. 1A, B). Early enhancement of the portal vein was seen during the arterial phase. During the portal venous phase, the affected portion of the pancreas demonstrated homogeneous enhancement that was higher in attenuation relative to the unaffected portion of the pancreas. The lesion became isodense relative to the normal pancreatic parenchyma during the delay phase, making it difficult to define the lesion at this phase. These features were suggestive of a pancreatic AVM.
In addition, the CT obtained during the arterial phase also demonstrated a dissecting flap in the proximal SMA (Fig. 1D). The dissection began at 1.5 cm from the origin of the SMA and it spared the orifice, and the dissection continued over a 6 cm-segment, where the true lumen gave off a side branch. The false lumen was partially thrombosed (Fig. 1C). There were no findings on CT to suggest bowel ischemia.
Further imaging assessment with MRI was undertaken and the pancreatic AVM was seen as a tangle of tubular structures that demonstrated a characteristic signal void on the T2-weighted image, providing further confidence in the diagnosis (Fig. 1E). After intravenous injection of a paramagnetic contrast agent, the tortuous vascular structures became hyperintense during the early arterial phase (Fig. 1F). The SMA dissection also showed interesting features on MRI. On the T2-weighted image, while the true lumen demonstrated signal void due to fast flowing blood, the false lumen showed high signal intensity due to slower blood flow (Fig. 1G). The proximal portion of the false lumen was not thrombosed and it showed enhancement during the early arterial phase (Fig. 1H).
The true incidence of pancreatic AVM is unknown and its etiology is not fully understood. It is most commonly congenital in origin, and it arises from anomalous differentiation in the rudimentary plexus of primordial blood vessels, but pancreatic AVM may also be acquired; it can develop secondarily to trauma, pancreatitis, pancreatic tumors or even liver cirrhosis (12345). Pancreatic AVM most commonly involves the head of the pancreas, but this may be found in other portions of the pancreas. The various complications of pancreatic AVM include gastrointestinal bleeding, hemosuccus pancreaticus, pancreatitis and portal hypertension, and the latter can eventually lead to hepatic failure (12467). In the previous literature, pancreatic AVMs were occasionally misinterpreted on CT as hypervascular tumors and this necessitated further evaluation with angiography (467). However, the development of MDCT has brought about recent advances in CT angiography, and these advances have allowed accurate visualization of the visceral vasculature in the abdomen without the invasiveness of conventional angiography. In our patient, MDCT during the arterial phase allowed accurately depicting the tortuous vascular network in the pancreas so that it could be differentiated from hypervascular tumors without any difficulty. MPR and the volume-rendered techniques provided better visualization of the feeding arteries and draining veins associated with the pancreatic AVM. MRI added further confidence to making the diagnosis for this patient becuase MRI demonstrated the characteristic signal void structures that suggested the high-flow vascularity, which was consistent with that of an AVM. The past reports in the literature have described the usefulness of Doppler US for making the diagnosis of pancreatic AVM (46).
MDCT and MRI also accurately demonstrated the presence of an isolated SMA dissection in this patient. The recent advances in these modalities have allowed early detection of isolated spontaneous SMA dissection, even in asymptomatic patients, and so this contributed to good outcomes (89). The SMA is the most common visceral artery in the abdomen to be affected by spontaneous dissection and most of the cases are idiopathic, although rare associations with cystic media necrosis, fibromuscular dysplasia, arteriosclerosis, hypertension, trauma and connective tissue disease have been described (10). An association of SMA dissection with pancreatic AVM has never been reported in the past. Acute symptoms such as abdominal pain are thought to be due to acute compression of the true lumen by the rapidly enlarging false lumen, which compromises the blood flow to the distal SMA (10).
Although CT angiography by itself is not sufficient for assessing the hemodynamics, which can be done with conventional angiography, the SMA distal to the site of dissection and the features suggestive of bowel ischemia are readily visible on CT, as was shown in our patient. Suzuki et al. have summarized the various CT features of SMA dissection, and these are: 1. thrombosis of the false lumen or an intramural hematoma, 2. an intimal flap, 3. an enlarged diameter of the SMA, 4. increased attenuation of the fat around the SMA and 5. hematoma in the mesentery with hemorrhagic ascites (9). We have found that MRI also accurately depicted the dissection flap within the proximal SMA lumen, and that the true and false lumens can be differentiated by the discrepancy of signal intensities within each lumen. The true lumen of the SMA demonstrated a signal void due to preserved blood flow, while the false lumen was hyperintense due to the sluggish blood flow. A hypointense thrombus was noted in the blinded end of the false lumen.
There is ongoing debate as to how to manage patients with either pancreatic AVM or SMA dissection.If no treatment for a pancreatic AVM is undertaken, it may gradually increase in size and this will eventually lead to portal hypertension that cannot be corrected even after surgery. Therefore, surgical excision of the AVM or either partial or total pancreatectomy has been recommended at the time of discovery, even in asymptomatic patients (3). As suggested in the report by Rezende et al. transarterial embolization (TAE) may be considered as a safe and effective nonoperative approach, but complete embolization of a pancreatic AVM may sometimes be difficult due to the presence of multiple feeding vessels. Furthermore, complications associated with TAE have been reported such as the formation of new collateral vessels that result in repeated hemorrhage and portal hypertension, and also unwanted embolization of the bowel and pancreas leading to ischemic injuries (57). However, TAE has been shown to be effective for controlling bleeding in the patients who are contraindicated for surgery and TAE may also be considered preoperatively in order to reduce the portal venous pressure and lower the risk of intraoperative or postoperative hemorrhage (345).
As for SMA dissection, it has been suggested that asymptomatic patients should be managed conservatively (89). Anticoagulation can help prevent thrombosis of the lumens and the subsequent impaired distal flow, as well as possible emboli, although it cannot halt progression of dissection and aneurysmal enlargement of the SMA. For patients who are undergoing conservative treatment, close follow-up, both clinically and radiologically, is mandatory (9). Persistent symptoms despite coagulation, clinical signs of mesenteric ischemia and such imaging findings at follow-up as an increasing size of the aneurysmal dilatation of the SMA and thrombosis of the lumen of the SMA should spur the clinician to consider performing percutaneous endovascular intervention or surgery. Leung et al. report the first case of percutaneous endovascular treatment by stent placement in a patient who showed a suboptimal response to conservative therapy, but only a few successful cases of stent placement for SMA dissection has been described in literature since that report. The aim of endovascular stent deployment is to prevent extension of the dissection by ensuring recanalization of the artery and obliterating the false lumen (8).
Although there is no evidence to suggest any causal relationships between pancreatic AVM and SMA dissection and their etiologies have yet to be explained, this report describes the MDCT and MRI features of a rare case of pancreatic AVM and isolated SMA dissection that were discovered together in one patient.
Figures and Tables
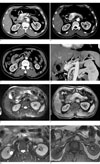
Fig. 1
The MDCT and MRI findings of a 57-year-old man with epigastric pain.
A. The axial scan during the arterial phase shows early-enhancing tortuous, dilated mass of vasculature (curved arrow) in the body of the pancreas. A dissecting flap (straight arrow) is seen within the SMA, beginning just distal to its origin from the aorta.
B. Early enhancement of the portal vein (curved arrow) is seen during the arterial phase.
C. The false lumen (within the broken lines) is seen as the low-attenuation filling defect that is due to thrombus.
D. The oblique coronal MPR image provides a clearer image of both the pancreatic AVM (curved arrow) and the SMA dissection (straight arrow).
E. On the fat-suppressed T2WI, the pancreatic AVM (curved arrow) demonstrates the characteristic signal void.
F. The AVM (curved arrow) shows marked enhancement on the contrast-enhanced T1WI.
G. The fat-suppressed T2WI of the proximal SMA reveals a discrepancy in the signal intensities of the true and false lumens (within the broken lines). The true lumen demonstrates a signal void due to high blood flow, while the false lumen shows hyperintensity due to the slower blood flow.
H. The contrast-enhanced T1WI demonstrates enhancement of both the true and false lumens of the dissected SMA (within the broken lines).
References
1. Halpern M, Turner AF, Citron BP. Hereditary hemorrhagic teleangiectasia. an angiographic study of abdominal visceral angiodysplasia associated with gastrointestinal hemorrhage. Radiology. 1968; 90:1143–1149.
2. Bauersfield SR. Dissecting aneurysm of the aorta: a presentation of fifteen cases and a review of the recent literature. Ann Intern Med. 1947; 26:873–889.
3. Hosogi H, Ikai I, Hatano E, Taura K, Fujii H, Uamamoto Y, et al. Pancreatic arteriovenous malformation with portal hypertension. J Hepatobiliary Pancreat Surg. 2006; 13:344–346.
4. Yoon JH, Han SS, Cha SS, Lee SJ. Color Doppler ultrasonography of a pancreatic arteriovenous malformation. J Ultrasound Med. 2005; 24:113–117.
5. Nishiyama R, Kawanishi Y, Mitsuhashi H, Kanai T, Ohba K, Mori T, et al. Management of pancreatic arteriovenous malformation. J Hepatobiliary Pancreat Surg. 2000; 7:438–442.
6. Kurosaki M, Hattori K, Minato Y, Shiiqai T, Ohashi I, Umehara I, et al. Asymptomatic arteriovenous malformation of the pancreas: demonstration by Doppler ultrasonography and magnetic resonance imaging. Dig Dis Sci. 1993; 38:1342–1346.
7. Rezende MB, Bramhall S, Hayes T, Olliff S, Buckels JA, Candinas D, et al. Pancreatic arteriovenous malformation. Dig Surg. 2003; 20:65–69.
8. Leung DA, Schneider E, Kubik-Huch R, Marincek B, Pfammatter T. Acute mesenteric ischemia caused by spontaneous isolated dissection of the superior mesenteric artery: treatment by percutaneous stent placement. Eur Radiol. 2000; 10:1916–1919.
9. Sparks SR, Vasquez JC, Bergan JJ, Owens EL. Failure of nonoperative management of isolated superior mesenteric artery dissection. Ann Vasc Surg. 2000; 14:105–109.
10. Sagiuchi T, Asano Y, Yanaihara H, Aoki Y, Woodhams R, Hayakawa K. Three-dimensional CT in isolated dissecting aneurysm of the superior mesenteric artery: a case report. Radiat Med. 2001; 19:271–273.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download