Acute renal cortical necrosis (RCN) is a rare condition of partial or complete necrosis of the renal cortex, and this usually spares a thin tissue rim under the capsule and a thicker layer adjacent to the corticomedullary junction. It typically evolves bilaterally, although a few unilateral RCN cases have been reported in patients with unilateral hydronephrosis or arterial stenosis. We encountered a case of bilateral RCN, including limited RCN in a kidney with unilateral hydronephrosis, following left sapingo-oophorectomy for treating the patient's ovarian cancer. We report here the MDCT finding of bilateral RCN, including limited RCN in a hydronephrotic kidney.
Case Report
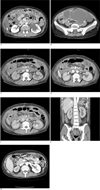
A 44-year-old woman was admitted to our hospital because of abdominal pain she had experienced over a three-month period. She had a history of total hysterectomy and right ovarian resection for an ovarian tumor. Upon medical examination, a mass was found in the left lower quadrant of the abdomen. A laboratory test showed a markedly elevated CA-125 level at about 3,800 U/L, with other test results being unremarkable. A 64-slice CT scan (Aquillion, Toshiba, Tokyo, Japan) for the entire abdominopelvic cavity was performed. The CT scanning parameters included a reconstruction interval of 3 mm, a rotation time of 0.6 seconds, a table speed of 41.7 mm/rotation, 120 kV and 200 mAs. The transverse CT images (the corticomedullary, nephrographic and excretory phases) were obtained 35, 75 and 180 seconds after the intravenous injection of non-ionic contrast material (120 mL, Ultravist 300, Bayer Schering Pharma, Berlin, Germany) at a rate of 2.8 mL/sec. The multiplanar reformatted coronal images were reconstructed from the transverse images. The preoperative CT images (Figs. 1A, B) revealed an approximately 12.5 × 12 × 7.5 cm multi-septated cystic mass in the left pelvic cavity, which had displaced the uterus and bladder to the right anterior side and obstructed the left ureter. Ascites fluid was found in the abdominopelvic cavity, and the presence of multiple implants and a streaky image density were indicative of extensive peritoneal carcinomatosis. There was hydroureteronephrosis at the left kidney, which was probably due to left distal ureter invasion by the mass.
Three days after preoperative CT examination, the patient underwent left sapingo-oophoretomy and total omentectomy. The left adnexal mass was determined by pathologic exam to be a serous papillary adenocarcinoma. On the third postoperative day, the BUN/Cr level had risen to 32/4.7, and it continued to rise gradually to 70/12.1 on the tenth postoperative day. Abdominopelvic CT was performed on the ninth postoperative day in order to evaluate the possible causes of this postoperative complication (Figs. 1C-F), and the CT showed decreased parenchymal enhancement in both kidneys. The right kidney exhibited a non-enhancing cortical band with preserved enhancement in the subcapsular portion, the inner cortex and the medullary portion, suggesting RCN. The low-density cortical band was delineated in all three phases, but this was most prominent in the corticomedullary phase. The left kidney showed improved hydronephrosis and multiple areas of subtle cortical low density bands with generalized reduced parenchymal enhancement, suggesting a limited form of RCN. The patient underwent several hemodialysis treatments, and the BUN/Cr level gradually returned to the normal range and she was discharged. After three months, the patient was readmitted for chemotherapy, and abdominopelvic CT was performed. The follow-up MDCT (Fig. 1G) showed a decrease in the size of the right kidney with a delayed parenchymal transit time.
Discussion
The pathologic changes of RCN range from limited or patchy necrotic changes to complete cortical necrosis. In its most complete form, almost the entire cortex is necrotic, and this usually spares the corticomedullary junction and a thin rim of subcapsular tissue. The degree of pathologic damage seems to be dependent on the length and severity of the insult (1). The characteristic CT findings for RCN include a thin band of low attenuation in the cortex that excludes the thin rim of the subcapsular cortex, the thicker juxtamedullary cortex and the medulla, and this all well correlates with the histopathologic findings (2).
The common precipitating causes of RCN are complications of pregnancy and abruptio placenta. Today, nonobstetric causes are increasing in their relative incidence with the development of obstetric care. Other reported causes of RCN are sepsis, shock, gastrointestinal bleeding, trauma, burns, renal transplantation, transfusion reaction and snake bites. Hemolytic uremic syndrome (HUS) and thrombolytic thrombocytopenia purpura (TTP) are sometimes complicated by RCN (3). In the present case, the cause of RCN was thought to be postoperative shock, but this is uncertain.
The pathogenenesis of RCN remains unclear, although vasospasm and disseminated intravascular coagulation have been proposed as primary causes. Acute RCN results from selective arterial spasm of the cortical vasculature with the unique involvement of only part of the renal vasculature, i.e., the renal interlobular and afferent vessels with sparing the main renal arteries and branches up to the arcuate level. The subcapsular nephrons are usually spared from infarction in acute RCN due to the collateral circulation from extra-renal arteries, and the juxtamedullary cortex survives because of the blood supply from the arcuate artery (4). Most of the previously reported cases of RCN have been bilateral. Unilateral renal cortical necrosis is very rare with only nine reported cases, of which seven showed obstruction of the ureter on the protected side and the other two cases had renal artery stenosis (5).
Prolonged ureteral obstruction is likely to reduce the renal blood flow. It is possible that this may somehow reduce the number of glomerular thrombi, although it is difficult to understand their complete absence. It has been reported that hydronephrosis changes the glomerular hemodynamics by opening anatomical shunts (6), which is a possibility compatible with the complete absence of thrombi. According to a recent report, hydronephrosis may produce protective agents such as insulin-like growth factor (IGF-1) (7) and prostaglandin E2 (PGE-2) (8). PGE-2 can counteract the vasoconstrictor responses of the afferent arterioles, and it may thereby modulate the actions of angiotensin II (9), thus providing protection against renal ischemia.
In the present case, the RCN involved both kidneys, although they differed in severity with a limited form in the kidney with hydronephrosis. On contrast-enhanced imaging MDCT, a subtle but definite thin rim with cortical low density was observed in the hydronephrotic kidney. Follow-up MDCT performed three months later revealed atrophy of the severely affected kidney.
CT with a 64-section multidetector provides reliable assessments of regional renal perfusion, the tubular dynamics and the GFR, and it shows good agreement with the previously validated electron-beam CT measurements. The minimal invasiveness of this tool and its increasing availability make multidetector CT a powerful aid for the study of the kidney under both physiologic and pathologic conditions (10). MDCT with a thinner scan thickness and three dynamic phases may facilitate the observation of subtle CT findings in the limited form of RCN.
In the present case, the low density cortical bands of RCN in the right kidney were widest in the corticomedullary phase. Also, the limited form of RCN in the left kidney was more distinctly seen in the corticomedullary phase. It means that the early phase of the enhanced MDCT image could more clearly delineate the subtle low density band of the limited form of RCN. The coronal reconstruction image can show the RCN more sterically.
In the present case, we observed a thin subcapsular low density rim in the cortex on an enhanced MDCT image of the hydronephrotic kidney, suggesting limited RCN. There have been no previous case reports of RCN with the MDCT images. This disparity of RCN between the two kidneys is very rare condition. Thus, we report here on the MDCT finding of bilateral renal cortical necrosis, including limited RCN in a hydronephrotic kidney.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download