Abstract
A pulmonary pleomorphic carcinoma is a rare, poorly differentiated, primary malignancy. We report an atypical manifestation of this rare tumor, with the air crescent and CT halo signs.
Pulmonary pleomorphic carcinoma is rare, accounting for approximately 0.1-0.4% of all lung malignancies. These tumors usually present in symptomatic older male smokers as large peripheral masses, often with chest wall invasion and metastases at diagnosis (1). We recently experienced an atypical case of pleomorphic carcinoma with the air crescent and CT halo signs in a young woman without a history of smoking. The air meniscus and CT halo signs associated with pleomorphic carcinoma have not been previously reported. Here we present the radiology and pathology correlations as well as a review of the medical literature.
A 38-year-old woman was referred to our hospital with an abnormality on chest X-ray. The patient complained of a dry cough over the past few months. In addition, she had neither a history of smoking nor a significant medical history. Upon admission, her physical and laboratory findings were unremarkable. In addition, sputum studies for acid-fast bacilli, Aspergillus organisms, other pathological organisms, and malignant cells were negative. On the initial chest X-ray, a large mass was discovered in the right lower lobe of the lung with the air crescent (Fig. 1A). The chest CT showed a 6 cm sized sharply defined, homogenous mass with lobulation at the periphery of the right lower lobe of the lung (Fig. 1B). After contrast infusion, the mass enhanced inhomogeneously with a focal eccentric area of decreased enhancement. In the lung window setting, the mass was surrounded by ground glass attenuation (Fig. 1C). An interface between the ground glass attenuation and normal parenchyma was relatively sharply delineated. A crescent-shaped air space with a smooth inner surface was seen at the anterior aspect of the mass. The mass obstructed the anterior basal segmental bronchus, resulting in segmental atelectasis. There was no evidence of pleural effusion, chest wall invasion, lymphadenopathy, or distant metastasis. The differential diagnosis before surgery included sclerosing hemangioma with peritumoral hemorrhage, lung cancer arising from a cavity, and cavitary lung cancer. Aspergilloma was ruled out by the enhancement pattern of the mass on CT. A fiberoptic bronchoscopy and transbronchial washing cytology were performed, but the findings were inconclusive. The patient underwent a right lower lobectomy for both a definite diagnosis and treatment. The macroscopic examination showed a lobulated, yellowish mass with an eccentric cystic space. The microscopic examination revealed a pleomorphic carcinoma consisting of adenocarcinoma and giant cell carcinoma. Giant cell carcinoma was mainly located in the central portion of the mass, which was surrounded by adenocarcinoma with bronchioloalveolar carcinoma (BAC) features (Fig. 1D). The cystic space within the mass was lined by tumor cells, adenocarcinoma, and also contained some islets of adenocarcinoma components (Fig. 1E).
Pulmonary pleomorphic carcinoma is a subtype of carcinoma with pleomorphic, sarcomatoid, or sarcomatous components. Histologically, it is defined as a non-small cell lung carcinoma with neoplastic spindle and/or giant cells, having at least a 10% spindle cell or giant cell component (456). Pleomorphic carcinoma is common in older men and is strongly associated with a history of smoking. It usually presents as a large, peripheral, necrotic mass with upper lobe predilection, invading the chest wall (36).
Recently, two reports on the CT features as well as the radiological and pathological correlations of the tumors have been published. According to Kim et al. (7) in a recent study of 10 cases of pleomorphic carcinoma of the lungs, the tumors preferentially manifest as large peripheral masses with central low attenuation areas, frequently invading the pleura and chest wall. They concluded that low attenuation areas on contrast enhanced CT scans corresponding to areas of myxoid degeneration, necrosis, or hemorrhage on pathology.
In addition, Kim et al. (8) suggested that the CT features might be dominated by the epithelial component of the tumor after review of the CT and pathological findings of 30 cases of pleomorphic carcinoma. According to their study, pleomorphic carcinomas with adenocarcinoma or large cell components tend to be located in the peripheral zone. However, tumors with squamous cell components were located in the central zones. In their study, central necrosis was frequent but cavity formation in the tumor was uncommon for the pleomorphic carcinoma. The frequency of necrosis and tiny cavity formation was higher in the large and giant cell subtypes compared to others. Moreover, peritumoral areas of ground-glass attenuation were characteristics of the large and giant cell subtypes, and are associated with intra-alveolar macrophage aggregation and alveolar wall thickening with inflammatory cell infiltration and mild fibrosis on the pathology specimens.
In our case, the mass was initially thought to be a sclerosing hemangioma, because the mass was a well-defined contrast-enhancing juxtapleural mass in a young woman without a history of smoking. Moreover, the air crescent and CT halo signs, in our case, were characteristics consistent with sclerosing hemangioma, which were explained by repeated peritumoral bleeding with fibrosis and bronchial communication with the check valve phenomenon (9).
The radiology and pathology correlations, in our case, revealed that the air crescent represented the cystic changes associated with adenocarcinoma, without the evidence of communication with the airway. Moreover, the peripherally located bronchioloalveolar carcinoma was associated with ground glass attenuation. Kim et al. (8) reported that peritumoral ground glass attenuation was caused by inflammation. In our case, the ground glass attenuation, manifested by the bronchioloalveolar component of the pleomorphic carcinoma, was clearly demarcated with associated cystic changes; CT findings well known to be consistent with bronchioloalveolar carcinoma. Wang et al. (10) reported a case report of adenocarcinoma with an air crescent sign as observed in our case. They concluded that the emphysematous or cystic change between the tumor-infiltrating bands was caused by the infiltration of adenocarcinoma cells with the paracicatrical effect around the pulmonary parenchyma.
In summary, this unusual case of pleomorphic carcinoma with the air crescent and CT halo signs were associated with the adenocarcinoma and bronchioloalveolar carcinoma component of a pleomorphic carcinoma.
Figures and Tables
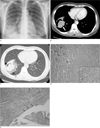 | Fig. 1Pleomorphic carcinoma in a 38-year-old woman.A. Initial chest PA reveals a thick-walled cavitary mass with an air crescent sign in the right lower lobe of the lung.
B. Axial contrast enhanced CT scan shows a 6 cm sized, lobulated, inhomogeneous enhancing mass located at the periphery of the right lower lobe of the lung (white thick arrow). Note the segmental atelectasis at the anterior basal segment, caused by the obstruction of the mass.
C. The CT image obtained using lung window settings shows a crescent-shaped air space (arrowhead) as well as peritumoral areas of ground glass attenuation (double thin black arrows). Note the relatively sharp interface between the ground glass attenuation and normal lung parenchyma.
D. Photomicrograph of the histopathological specimen shows solid tumor with multi-nucleated giant cells (inset) and adenocarcinoma mixed subtype with BAC in the surrounding parenchyma. (H & E, ×12) The peritumoral areas of ground glass attenuation represent the BAC component (arrows) of this tumor.
E. Photomicrograph shows the adenocarcinoma component of the tumor. Note the cluster of tumor cells within the cystic space. The wall of the cystic space is also lined with adenocarcinoma tumor cells (arrows). (H & E, ×12)
|
References
1. Rossi G, Cavazza A, Sturm N, Migaldi M, Facciolongo N, Longo L, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol. 2003; 27:311–324.
2. Kita Y, Nogimura H, Kato M, Hasegawa H, Nagayama M, Nishihara K, et al. Cavitating pleomorphic carcinoma of the lung; report of a case. Kyobu Geka. 2006; 59:959–961.
3. Chang YL, Lee YC, Shih JY, Wu CT. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer. 2001; 34:91–97.
4. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumors. Eur Respir J. 2001; 18:1059–1068.
5. Travis WD. Pathology of lung cancer. Clin Chest Med. 2002; 23:65–81.
6. Fishback NF, Travis WD, Moran CA, Guinee DG Jr, McCarthy WF, Koss MN. Pleomorphic (spindle/giant cell) carcinoma of the lung: a clinicopathologic correlation of 78 cases. Cancer. 1994; 73:2936–2945.
7. Kim TH, Kim SJ, Ryu YH, Lee HJ, Goo JM, Im JG, et al. Pleomorphic carcinoma of lung: comparison of CT features and pathologic findings. Radiology. 2004; 232:554–559.
8. Kim TS, Han J, Lee KS, Jeong YJ, Kwak SH, Byun HS, et al. CT findings of surgically resected pleomorphic carcinoma of the lung in 30 patients. AJR Am J Roentgenol. 2005; 185:120–125.
9. Takatani H, Ashizawa K, Kawai K, Kohno S. Pulmonary sclerosing hemangioma manifesting as a nodule with irregular air clefts on high resolution CT. AJR Am J Roentgenol. 2007; 189:W26–W28.
10. Wang LF, Chu H, Chen YM, Perng RP. Adenocarcinoma of the lung presenting as a mycetoma with an air crescent sign. Chest. 2007; 131:1239–1242.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download