Abstract
Multifocal central giant cell granulomas (CGCG) in the maxillofacial region are suggestive of systemic disease such as hyperparathyroidism or an inherited syndrome such as Noonan-like multiple giant cell lesion syndrome. Only 5 cases of multifocal CGCGs in the maxillofacial region without any concomitant systemic disease have currently been reported. We report here on an unusual case of 17-year-old man who presented with multifocal CGCGs of the bilateral posterior mandible and right maxilla and he was without any concomitant systemic disease.
Central giant cell granulomas (CGCG) of the jaws usually present as a painless lesion and a solitary radiolucent expansion is seen on the simple radiographs. This disease is most commonly diagnosed in the third decade of life, and females are twice as likely to be affected as males (1). The gnathic cases of giant cell granuloma have accounted for 1-7% of all the benign lesions in several previously reported oral surgical series. Jaw lesions affect women more frequently than men (2:1 ratio) (2). The presence of multiple CGCGs in the maxillofacial region is suggestive of hyperparathyroidism or a number of syndromes (34). To the best of our knowledge, only 5 cases of multifocal CGCGs without any concomitant systemic disease have currently been reported (45). We report here on a case of multifocal CGCGs in the maxillofacial region of a 17-year-old male and the patient was without any concomitant systemic disease.
A 17-year-old man presented with right nasal obstruction and rhinorrhea that he had suffered with for the previous five months. There was no history of prior trauma or surgery to the mandible. The clinical examination revealed a mass in the right posterior nasal cavity. We then performed computed tomography (CT) and magnetic resonance imaging (MRI) of the paranasal sinuses and nasal cavity.
The CT scan of the right maxilla with the bone window setting showed a relatively well defined, heterogeneous isodense mass with mineralized septa, expansile boney remodeling with cortical thinning of the medial and lateral walls of the antrum and opacification of the antrum and the posterior nasal cavity. The mass had eroded the adjacent bony structures, such as the anterior wall of the sphenoid sinus, and it extended to the surrounding spaces, such as the right maxillary sinus and the posterior nasal cavity. There were also bilateral osteolytic lesions with cortical expansion, boney thinning and mineralized septa in both sides of the posterior mandible (Fig. 1).
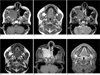
On MR imaging, multifocal lesions in the right maxilla and bilateral mandible showed low signal intensity on the T1-weighted images and heterogeneous low to intermediate signal intensity on the T2-weighted images. The mass showed intense enhancement after the administration of contrast material (Fig. 2).
Considering these imaging findings, we considered brown tumor of hyperparathyroidism, ameloblastoma, odontogenic myxoma and odontogenic fibroma as possible options for making the differential diagnosis.
The blood serum levels of parathyroid hormone, alkaline phosphatase and calcium were within normal limits (Table 1). There were no other bony lesions in the entire body.
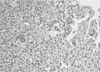
Biopsy was performed on the mass in the right maxillary sinus. Histologically, the mass showed a proliferation of multinucleated giant cells within a background of plump, ovoid and spindle-shaped mesenchymal cells. The giant cells contained only a few nuclei, up to dozen (Fig. 3). Thus, the pathologic diagnosis was central giant cell granuloma.
Jaffe in 1953 first described giant cell reparative granuloma (GCRG) as a benign lesion that affects the mandible and maxilla, and Jaffe suggested that this is a reactive response to intraosseous hemorrhage (6). It was initially termed giant cell reparative granuloma, yet these lesions are no longer believed to represent a reparative process (3).
The gnathic cases of GCRG are categorized based on their location as central (i.e., occurring in bone) or peripheral (i.e., occurring in the gingival soft tissues), and these gnathic cases of GCRG account for 1-7% of all the benign lesions in several previously reported oral surgical series. Jaw lesions affect women more frequently than they do men (2:1 ratio) (2). CGCG is more uncommon than peripheral giant cell granuloma, at a ratio 1:3 (4).
CGCG of the jaws usually presents as a solitary radiolucent expansion on simple radiograph and most of the cases are painless; CGCG is usually diagnosed in the third decade of life with females being twice as likely to be affected as males (1). CGCG is a benign lesion of bone with a variably aggressive nature (3). Some of these lesions are more destructive and they show a marked tendency to recur (1).
CGCGs are unifocal lesions that are generally seen in the anterior mandible, and to a lesser extent, in the other facial bones, hands and feet (5). Multicentric CGCGs have only rarely been reported in the medical literature (7). Most of the previously reported cases of multiple concurrent CGCGs were associated with some form of inherited syndrome or systemic disease (3).
Davis and Cassatly (7) reported on the first case of multicentric CGCGs in the absence of hyperthyroidism. To the best of our knowledge, only 5 cases of multifocal CGCGs without concomitant systemic disease have currently been reported in the English-language medical literature and there has been no reported case that occurred in the 2nd decade of life (1 male patient and 4 female patients; mean age 31.4 years; range: 23-41 years)(Table 2) (45).
The most commonly reported radiological finding of GCRG is lysis with expansile remodeling of the bone. The cortex around the lesion is thin with or without bone destruction (8). However, these radiological appearances are indistinguishable from other radiolucent bony lesions such as odontogenic cyst, aneurysmal bone cyst, ameloblastoma, odontogenic myxoma and odontogenic fibroma (2). The CT appearance of GCRG is not distinctive either. Cortical thinning and destruction, soft tissue extension and a lesion density similar to that of muscle are the usual findings of giant cell granuloma (8). Fibrous septa are a usual finding of giant cell granuloma (8). Mineralization can be seen in CGCG lesions, but this is uncommon and it is usually limited in extent (2). However, diffuse mineralized fibrous septa were noted in our case.
In the present case, the CT scan with a bone window setting showed relatively well defined, heterogeneous isodense multifocal masses with mineralized septa and expansile boney remodeling, and there was cortical thinning of the right maxilla and bilateral mandible. On the MR imaging, the multifocal lesions in the right maxilla and bilateral mandible showed low signal intensity on the T1-weighted images and heterogeneous low to intermediate signal intensity on the T2-weighted images without a cystic component. The mass showed intense enhancement after administration of contrast material. These radiologic findings of our patient are relatively typical findings of a usual CGCG (8). However, diffuse mineralized fibrous septa for this type of lesion have not been reported in the previous literature.
Multifocal CGCGs may be associated with hyperparathyroidism, cherubism or Noonan-like multiple giant cell lesion syndrome (1). The most difficult diagnosis to rule out when presented with multiple giant cell lesions is brown tumor of hyperparathyroidism (7). Our patient had no other biochemical or radiological evidence of hyperparathyroidism (1). Multiple giant cell lesions form part of Noonan-like syndrome, but our patient lacked any of the other features such as a short stature, low intelligence or developmental delay, ocular hyperteleorism, posteriorly angulated ears, pectus excavatum or pulmonary stenosis (1). Cherubism is an autosomal dominantly inherited condition with variable expressivity, and this is characterized by multiquadrant radiolucent lesions of the jaws. Clinically, cherubism most commonly manifests as a progressive and symmetrical enlargement of the mandible and/or the maxilla, and this is first noted between the ages of 1 and 4 years. There is no family history of similarly affected family members for cases of CGCG (3). Yet there are rare reported instances of synchronous multiple CGCGs of the maxillofacial region for which other causes or associated disorders have been excluded (3).
In conclusion, multifocal CGCGs in the maxillofacial region are suggestive of a systemic disease such as hyperparathyroidism or an inherited syndrome such as Noonan-like multiple giant cell lesion syndrome. The present study presents a case of multifocal, synchronous CGCGs in the bilateral mandible and right maxilla without any concomitant systemic disease. Although it is very rare, multiple CGCGs should be considered in the differential diagnosis of benign fibroosseous lesion that typically present as an expansile soft tissue mass of the maxillofacial region.
Figures and Tables
Fig. 1
A. The coronal CT scan with a bone window setting shows a relatively well defined, heterogeneous isodense mass (arrows) with diffuse mineralized septa (arrowheads) in the right maxilla.
B. The axial CT scan with a bone window setting shows expansile remodeling with cortical thinning of the medial and lateral walls of the maxillary antrum (arrowheads). The mass eroded the adjacent bony structures, such as the anterior wall of the sphenoid sinus (black arrow) and the mass extends to the right antrum and the posterior nasal cavity (white arrow).
C. The axial CT scan (below the level of B) shows multiple osteolytic lesions (arrows) with cortical expansion, bone thinning and mineralized septa (arrowheads) in the bilateral posterior mandible.
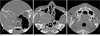
Fig. 2
A-F. The multiple masses (arrows) in the right maxillary sinus and bilateral posterior mandible show low signal intensity on the T1-weighted images (A, B), heterogeneous low to intermediate signal intensity on the T2-weighted images (C, D) and intense enhancement of the masses (E, F) after the administration of contrast agent.
References
1. Curtis NJ, Walker DM. A case of aggressive multiple metachronous central giant cell granulomas of the jaws: differential diagnosis and management options. Int J Oral Maxillofac Surg. 2005; 34:806–808.
2. Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radiographics. 2001; 21:1283–1309.
3. Edwards PC, Fox J, Fantasia JE, Goldberg J, Kelsch RD. Bilateral central giant cell granulomas of the mandible in an 8-year-old girl with Noonan syndrome (Noonan-like/multiple giant cell lesion syndrome). Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 99:334–340.
4. Junquera LM, Lupi E, Lombardia E, Fresno MF. Multiple and synchronodus peripheral giant cell granulomas of the gums. Ann Otol Rhinol Laryngol. 2002; 111:751–753.
5. Martins WD, de Oliveira Ribas M, Braosi AP, Machado MA, Lima AA. Multiple giant cell lesions of the maxillofacial skeleton. J Oral Maxillofac Surg. 2007; 65:1250–1253.
6. Morris JM, Lane JI, Witte RJ, Thompson DM. Giant cell reparative granuloma of the nasal cavity. AJNR Am J Neuroradiol. 2004; 25:1263–1265.
7. Smith PG, Marrogi AJ, Delfino JJ. Multifocal central giant cell lesions of the maxillofacial skeleton: a case report. J Oral Maxillofac Surg. 1990; 48:300–305.
8. Cakirer S. Quiz case. Giant cell reparative granuloma of the maxillary sinus. Eur J Radiol. 2002; 44:24–27.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download