Abstract
Background
Owing to its large molecular size, polyethylene glycol (PEG)-precipitable thyrotropin (TSH) can accumulate in the circulation, elevating TSH levels. PEG-precipitable TSH can be used to detect macro-TSH (mTSH) in serum. Our aim was to evaluate the prevalence of mTSH in patients who had undergone thyroidectomy for thyroid cancer.
Methods
Seventy-three thyroid cancer patients and 24 control subjects on levothyroxine (LT4) TSH-suppressive or replacement therapy were evaluated. Screening for mTSH was performed by adding PEG to serum in order to precipitate γ-globulin. A percentage of PEG-precipitable TSH ≥80% was considered suggestive of mTSH.
Results
No correlation between free-T4 (fT4) and TSH levels was found. PEG-precipitable TSH was 39.3%±1.9% in thyroid cancer patients and 44.1%±3.9% in controls. Macro-TSH was deemed to be present in one thyroid cancer patient and in two control subjects. Only in the thyroid cancer group was PEG-precipitable TSH found to be negatively correlated with fT4 concentration. No correlation was found between PEG-precipitable TSH and other clinical conditions in any patients.
Conclusion
The presence of mTSH seems to be a rare phenomenon in thyroid cancer. In some patients with low PEG-precipitable TSH, a reduction in LT4 dosage could be suggested. LT4 dosage adjusted to body weight is the main factor in maintaining TSH in a semi-suppressed or normal range. Evaluation of mTSH could be necessary in patients in whom a balance is required between adequate TSH suppression and the avoidance of unnecessary exogenous hyperthyroxinemia.
After primary therapies, adherence to follow-up examinations and the optimization of thyrotropin (TSH)-suppressive therapy with levothyroxine (LT4), respectively, contribute to ruling out tumor recurrence and improving quality of life. According to recent guidelines, LT4 dosage in thyroid cancer is age-, tumor stage-, and histology-dependent [1]. Therefore, the balance between necessary and unnecessary exogenous hyperthyroxinemia should always be considered in the management of thyroid cancer patients [2].
It is well known that that about 40% of patients taking LT4 for benign [3] or malignant [45] thyroid pathologies do not have serum TSH in the adequate range, and the risk of iatrogenic hypothyroxinemia and hyperthyroxinemia is always possible [2456]. The main causes of inappropriately high TSH levels are low LT4 dosages for body-weight and composition [7], drugs and foods which interfere with LT4 absorption [58], common gastrointestinal disorders [8], bariatric surgery [9], and pregnancy [10]. Rarer causes of increased TSH that mimic subclinical hypothyroidism are human anti-mouse antibodies that interfere with immunoassay systems [11] and the macro-molecular complex of TSH and anti-TSH immunoglobulin (macro-TSH [mTSH]) [12].
Macro-TSH displays a large molecular size on gel filtration chromatography (>150 kDa), and has been described in several case reports in recent years [131415]. As in the case of macro-prolactin [16], the bioactivity of mTSH may be low, and mTSH may accumulate in the circulation, causing spurious elevated TSH levels [13141516]. More recent papers from Hattori et al. [171819] reported that (1) mTSH exhibited laboratory features similar to those of subclinical hypothyroidism [17]; (2) that there were no commercial TSH immunoassay platforms that do not cross with mTSH [18]; and (3) that mTSH may persist in patients for a long time [19]. According to these authors, the incidence of subclinical hypothyroidism might be overestimated, owing to the presence of mTSH in sera, and screening for mTSH should be performed before hormone replacement therapy is initiated in subclinical hypothyroidism [17].
To our knowledge, studies on mTSH in other cohorts of patients are lacking. Recently, Kadoya et al. [20] reported that an increase in serum mTSH is associated with poor sleep quality in patients with cardiovascular risk factors and, surprisingly, that mTSH is present even in patients with normal TSH levels and is regulated by different mechanisms from those regulating free-TSH. No data are available in patients with a history of thyroid carcinomas, in whom LT4 therapy must be carefully tailored in order to prevent under-treatment or unnecessary over-treatment.
The aim of our single-center study was to evaluate the prevalence of mTSH in a cohort of patients who had undergone thyroidectomy for thyroid cancer and, as a control group, in patients who had undergone surgery for benign thyroid pathology. As stated by Hattori et al. [17] with regard to subclinical hypothyroidism, our main interest was to determine whether, in patients on chronic LT4 therapy, mTSH screening may be justified when higher than expected LT4 dosages are needed in order to achieve the therapeutic target of serum TSH.
Outpatients with a history of thyroid cancer (study group) and outpatients with post-surgical thyroidectomy for benign pathology (control group), over 18 years of age, were recruited in 2016 for the study of mTSH during their annual follow-up examination at our Thyroid Cancer Unit. Written informed consent to participate in the study was obtained from all patients. Data from 73 study group patients and 24 control group patients were evaluated. All subjects were on chronic LT4 treatment in order to reach age- and risk-adjusted TSH levels in the study group and to control post-surgical hypothyroidism in the control group. Brand LT4 in various formulations (tablets, soft gel, liquid solution) was taken on waking, in a fasting condition, 30 minutes before breakfast [5]. Some clinical data are reported in Table 1. The majority of thyroid cancers were papillary thyroid cancer (85% of total) with a low-risk stage on diagnosis. Total thyroidectomy was the primary treatment in all but five thyroid cancer patients (93%) and in all but three control subjects (88%). Ablative radioiodine therapy was undertaken in 67% of patients with thyroid cancer of follicular origin. At the time of the study, positive thyroid autoantibodies were found in two study group patients and one control group patient. In all but three cases (two medullary thyroid carcinomas; one papillary thyroid carcinoma) calcitonin (Ct) or thyroglobulin (Tg) levels were indicative of cure (96%). Patients who had undergone more conservative treatments were regarded as cured on the basis of more than 5 years' stability of their clinical and biochemical status.
Clinical examination comprised neck sonography, body weight (b.w.) evaluation, and pharmacological anamnesis. At the time of examination, which was performed between 2:00 PM and 4:00 PM, blood samples for the study were drawn. Free-T4 (fT4) and TSH levels were evaluated by means of electro-chemiluminescence immunoassays. Methods were optimized on an automated analytic platform (Cobas c701, Roche Diagnostics, Mannheim, Germany). Normal ranges are 12.0 to 22.0 pmol/L for fT4 and 0.3 to 4.2 mIU/L for TSH. The functional sensitivity of the TSH assay is 0.03 mIU/L, with intra- and inter-assay coefficients of variation of 3% and 7%, respectively. Tg and anti-Tg antibody (TgAb) were evaluated as previously reported [5]. Serum Ct was assayed by means of chemiluminescence immunoassay (Dia Sorin, Saluggia, Italy); in our laboratory the upper limit of the normal Ct range is 10 ng/L.
Macro-TSH was screened by adding polyethylene glycol (PEG, 25%; 0.2 mL) to serum (0.2 mL) in order to precipitate γ-globulin fractions. The values of TSH in the supernatant and 1:2 diluted sera were used for the analysis. In accordance with the method of Hattori et al. [171819], the PEG-precipitable TSH (%), which represents the concentration of mTSH, was calculated as follows: (serum TSH–TSH recovered after PEG)/serum TSH×100. Arbitrarily, a PEG-precipitable TSH (%) value ≥80% was considered suggestive of mTSH in serum.
GraphPad version 6.0 software (GraphPad, San Diego, CA, USA) was used for statistical analysis. To compare continuous data, the Mann-Whitney test was used. Percentages were compared by means of Fisher exact test. Correlations were evaluated by means of the Spearman test. Data are reported as mean±standard error of mean (SEM) if not otherwise reported. Significance was set at P≤0.05. Data collection and subsequent analysis were performed in compliance with the Helsinki Declaration and approved by the University of Genoa Ethics Committee.
The study and control groups were similar in age and male/female distribution. The length of time since surgery was significantly greater in the study group than in the control group (P=0.002). In keeping with the histological diagnosis, the dosage of LT4 therapy was slightly but significantly higher and TSH levels were slightly but significantly lower (P=0.005 for both) in the study group than in controls (Table 1). No correlation between fT4 and TSH levels was found in either group (Fig. 1). Only few subjects showed inadequate TSH levels for their clinical condition (study group, 12%; control group, 8%). Fig. 2 reports the distribution of PEG-precipitable TSH (%) values in both groups. In both groups, mTSH showed a normal distribution, ranging from 0% to 92%. On average, PEG-precipitable TSH was 39.3%±1.9% in the study group and 44.1%±3.9% in the control group (P=0.28). According to our arbitrary criteria, mTSH was deemed to be present in one female patient (75 years old) with a history of low-risk papillary thyroid carcinoma, who was on 1.3 µg/kg b.w. of LT4 to maintain TSH at a level of 1.77 mIU/L. This patient had undergone surgery alone 2 years earlier; we increased the dosage of LT4 to 1.5 µg/kg b.w. in order to maintain TSH levels in the 0.55 to 0.69 mIU/L range. In this patient, hypertension was the only other medical complaint. In two control subjects (one 60-year-old female and one 67-year-old male) with mTSH, thyroidectomy had been performed 5 and 2 years earlier for a follicular nodule and for laryngeal cancer, respectively. LT4 was administered at a dosage of 1.3 and 1.4 µg/kg b.w., respectively, and TSH levels were 0.47 and 0.30 mIU/L. The male control had hyperthyroxinemia (fT4, 25.3 pmol/L), while in the female control fT4 was 17.9 pmol/L. In both subjects, anti-hypertensive therapies were ongoing; the male was also taking calcium carbonate, which can interfere with LT4 absorption. In both subjects, a few adjustments to LT4 dosage were made in order to maintain TSH levels in the 0.31 to 0.94 mIU/L to 0.30 to 0.90 mIU/L ranges, respectively. In all subjects with a suspected history of mTSH, laboratory data were negative for rheumatoid arthritis.
Only in the study group was PEG-precipitable TSH (%) found to be negatively (P=0.03) correlated with fT4 concentration (Fig. 3). In neither group was any correlation found between PEG-precipitable TSH (%) and age, sex, body mass index, years since thyroidectomy, tumor stage, radioiodine therapy, Tg levels, TgAb status, b.w.-related LT4 dosage, other concomitant drugs or TSH levels.
The prevalence of macro-prolactin in patients with hyperprolactinemia ranges about from 10% to 30%, and it has been reported that this variability is dependent on the immunoassay platforms used [21]. The prevalence of mTSH is not well known. Macro-TSH seems to be a heterogeneous entity which can be suspected when normal fT4 levels are associated to elevated TSH levels, and when substances that commonly interfere with TSH assays, including heterophilic antibodies, human anti-mouse/animal antibodies (HAMA) and rheumatoid factors have been excluded [22]. Macro-TSH can also occur in patients with TSH concentrations within reference ranges [23]. To date, the bulk of data on mTSH prevalence have been provided by studies by Hattori et al. [171819]. On searching for mTSH in 681 sera from patients with subclinical hypothyroidism, and using a cut-off of PEG-precipitable TSH greater than 75%, these authors found a prevalence of mTSH of 1.6% after gel-filtration [17]. Subsequently, in 1901 sera from subclinical hypothyroidism patients, in which the presence of HAMA had been excluded, the prevalence of mTSH decreased to 0.8% [18]. A similar prevalence (0.6%) was reported by Mills et al. [24] in a study conducted on 495 sera with TSH levels above 10 mIU/L.
No data on cohorts of thyroidectomy patients are available. In our study, PEG-precipitable TSH was 39.3%±1.9% in patients who had undergone total thyroidectomy for thyroid cancer and 44.1%±3.9% in similarly treated patients with a final histological diagnosis of benignity. These percentages seem to be somewhat lower than those reported in 681 samples from subjects with a clinical diagnosis of subclinical hypothyroidism (from 21% to 100%; on average, 63%) [17] and in 10 samples from subjects with presumably normal thyroid function (from 58% to 79%) [15]. On the other hand, mTSH distribution seems to be quite normal in our sera, as in the study by Hattori et al. [17]. In our whole group, according to the arbitrary cut-off of ≥80%, the prevalence of mTSH (3.1%) was higher than that reported in subclinical hypothyroidism [171824]. It is not clear whether this difference is due to the different cohort of patients or to the lack, in our study, of gel-filtration or HAMA examinations. However, the significant negative correlation seen between TSH after PEG-precipitable evaluation and fT4 concentrations in the study group, but not between TSH and fT4 levels before PEG precipitation, indicates that, in some patients, LT4 is not adequately titrated in relation to both elevated free-TSH and fT4. Literature data indicate that there is an increased need for LT4 in post-thyroidectomy patients, and that a wide range of daily LT4 regimens are available [2526]. This increase has been ascribed to the need to replace direct glandular triiodothyronine production, which is no longer available in post-surgical hypothyroid patients. Our data demonstrate that, in assessing the adequacy of LT4 dosage after total thyroidectomy in thyroid cancer patients, the individual balance of fT4 and mTSH must also be considered.
In their review of published case reports, Loh et al. [15] reported that the majority of patients with mTSH were females without symptoms of thyroid disorders, except for one with clinical features of hyperthyroidism. Hattori et al. [18] stated that mTSH is predominant in samples from elderly patients and that autoimmune mechanisms might be involved in the generation of mTSH [17]. On the other hand, some patients with mTSH have anti-Tg, anti-TSH receptors, and anti-thyroperoxidase antibodies [15]. Although our population consisted of middle-aged and elderly patients, no correlation was noted between mTSH and age or TgAb status. Moreover, no correlation was found in the study group between mTSH and disease stage or previous radioiodine therapy. In our opinion, more data are needed in order to attribute mTSH generation to age, sex, or autoimmunity.
It has been suggested the screening for TSH in subclinical hypothyroidism is necessary before LT4 therapy is initiated [1718]. However, this seems routinely impracticable; the same can probably be said in the case of thyroid cancer patients. Moreover, the detection of mTSH must be undertaken in particular conditions in surgical hypothyroid patients (present data), such as in patients with sleep disorders [20].
Our study has several limitations: (1) both the study and control groups comprised few subjects; (2) no other laboratory procedures were performed after PEG-precipitation; (3) TSH was assayed by means of only one analytical platform; and (4) the cut-off of PEG-precipitable TSH was set arbitrarily.
In conclusion, free-TSH levels after PEG precipitation are on average 40% lower than serum TSH levels in hypothyroid subjects with or without a history of thyroid carcinoma. The presence of mTSH seems to be a rare phenomenon in our patients. Patients suspected of having mTSH must be followed up for a long time, owing to the hypothesized long persistence of the immunoglobulin G-TSH complex [19]. In some patients with low PEG-precipitable TSH (%), a reduction in LT4 dosage could be suggested. Moreover, LT4 dosage adjusted to b.w. is the main factor in maintaining TSH in a semi-suppressed or normal range. More serum samples need to be evaluated in order to strike a balance between adequate TSH suppression and the avoidance of unnecessary long-term exogenous hyperthyroxinemia.
Figures and Tables
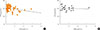 | Fig. 1Correlation between free-T4 (fT4) and thyrotropin (TSH) levels in (A) study (n=73; Spearman correlation coefficient [Sr], −0.20; P=0.09) and (B) control (n=24; Sr, −0.20; P=0.35) groups. |
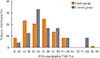 | Fig. 2Distribution of polyethylene glycol (PEG)-precipitable thyrotropin (TSH) ratios in study (orange columns) and control (gray columns) groups. |
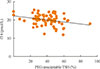 | Fig. 3Correlation between free-T4 (fT4) levels and polyethylene glycol (PEG)-precipitable thyrotropin (TSH) ratio (%) in the study group (n=74; Spearman correlation coefficient, −0.25; P=0.03). |
Table 1
Clinical Data of Study and Control Group Subjects

Values are expressed as mean±SEM or number (%). Tumor staging was done according to the American Joint Committee on Cancer (AJCC) 7th edition, AJCC.
PTC, papillary thyroid cancer; FvPTC, follicular variant of PTC; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; LT4, levothyroxine; b.w., body weight; fT4, free-T4; TSH, thyrotropin.
References
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.
2. Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid. 2010; 20:135–146.
3. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000; 160:526–534.
4. Lam E, Strugnell SS, Bajdik C, Holmes D, Wiseman SM. Limited adequacy of thyroid cancer patient follow-up at a Canadian tertiary care centre. Can J Surg. 2013; 56:385–392.
5. Giusti M, Mortara L, Machello N, Monti E, Pera G, Marenzana M. Utility of a liquid formulation of levo-thyroxine in differentiated thyroid cancer patients. Drug Res (Stuttg). 2015; 65:332–336.
6. Batrinos ML. The problem of exogenous subclinical hyperthyroidism. Hormones (Athens). 2006; 5:119–125.
7. Santini F, Pinchera A, Marsili A, Ceccarini G, Castagna MG, Valeriano R, et al. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J Clin Endocrinol Metab. 2005; 90:124–127.
8. Skelin M, Lucijanic T, Amidzic Klaric D, Resic A, Bakula M, Liberati-Cizmek AM, et al. Factors affecting gastrointestinal absorption of levothyroxine: a review. Clin Ther. 2017; 39:378–403.
9. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010; 11:41–50.
10. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017; 27:315–389.
11. Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem. 1999; 45:942–956.
12. Gurnell M, Halsall DJ, Chatterjee VK. What should be done when thyroid function tests do not make sense? Clin Endocrinol (Oxf). 2011; 74:673–678.
13. Sakai H, Fukuda G, Suzuki N, Watanabe C, Odawara M. Falsely elevated thyroid-stimulating hormone (TSH) level due to macro-TSH. Endocr J. 2009; 56:435–440.
14. Rix M, Laurberg P, Porzig C, Kristensen SR. Elevated thyroid-stimulating hormone level in a euthyroid neonate caused by macro thyrotropin-IgG complex. Acta Paediatr. 2011; 100:e135–e137.
15. Loh TP, Kao SL, Halsall DJ, Toh SA, Chan E, Ho SC, et al. Macro-thyrotropin: a case report and review of literature. J Clin Endocrinol Metab. 2012; 97:1823–1828.
16. Samson SL, Hamrahian AH, Ezzat S. AACE Neuroendocrine and Pituitary Scientific Committee. American College of Endocrinology (ACE). American Association of Clinical Endocrinologists, American College of Endocrinology Disease state clinical review: clinical relevance of macroprolactin in the absence or presence of true hyperprolactinemia. Endocr Pract. 2015; 21:1427–1435.
17. Hattori N, Ishihara T, Yamagami K, Shimatsu A. Macro TSH in patients with subclinical hypothyroidism. Clin Endocrinol (Oxf). 2015; 83:923–930.
18. Hattori N, Ishihara T, Shimatsu A. Variability in the detection of macro TSH in different immunoassay systems. Eur J Endocrinol. 2016; 174:9–15.
19. Hattori N, Ishihara T, Matsuoka N, Saito T, Shimatsu A. Anti-thyrotropin autoantibodies in patients with macro-thyrotropin and long-term changes in macro-thyrotropin and serum thyrotropin levels. Thyroid. 2017; 27:138–146.
20. Kadoya M, Koyama S, Morimoto A, Miyoshi A, Kakutani M, Hamamoto K, et al. Serum macro TSH level is associated with sleep quality in patients with cardiovascular risks: HSCAA Study. Sci Rep. 2017; 7:44387.
21. Smith TP, Suliman AM, Fahie-Wilson MN, McKenna TJ. Gross variability in the detection of prolactin in sera containing big big prolactin (macroprolactin) by commercial immunoassays. J Clin Endocrinol Metab. 2002; 87:5410–5415.
22. Ismail AA, Walker PL, Barth JH, Lewandowski KC, Jones R, Burr WA. Wrong biochemistry results: two case reports and observational study in 5310 patients on potentially misleading thyroid-stimulating hormone and gonadotropin immunoassay results. Clin Chem. 2002; 48:2023–2029.
23. Lewis EJ, Lim R, Joseph F, Ewins D, Goenka N, Bowles SA, et al. Recognising macro-TSH: a rare cause of inappropriately high TSH values. Clin Chem Lab Med. 2011; 49:Suppl. S421.
24. Mills F, Jeffery J, Mackenzie P, Cranfield A, Ayling RM. An immunoglobulin G complexed form of thyroid-stimulating hormone (macro thyroid-stimulating hormone) is a cause of elevated serum thyroid-stimulating hormone concentration. Ann Clin Biochem. 2013; 50(Pt 5):416–420.
25. Sukumar R, Agarwal A, Gupta S, Mishra A, Agarwal G, Verma AK, et al. Prediction of LT4 replacement dose to achieve euthyroidism in subjects undergoing total thyroidectomy for benign thyroid disorders. World J Surg. 2010; 34:527–531.
26. Del Duca SC, Santaguida MG, Brusca N, Gatto I, Cellini M, Gargano L, et al. Individually-tailored thyroxine requirement in the same patients before and after thyroidectomy: a longitudinal study. Eur J Endocrinol. 2015; 173:351–357.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download