1. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017; 27:1341–1346. PMID:
29091573.

2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID:
26462967.

3. Jung CK, Hong S, Bychkov A, Kakudo K. The use of fine-needle aspiration (FNA) cytology in patients with thyroid nodules in Asia: a brief overview of studies from the Working Group of Asian Thyroid FNA Cytology. J Pathol Transl Med. 2017; 51:571–578. PMID:
29073758.

4. Baek JH. Current status of core needle biopsy of the thyroid. Ultrasonography. 2017; 36:83–85. PMID:
28301922.

5. Pitman MB, Abele J, Ali SZ, Duick D, Elsheikh TM, Jeffrey RB, et al. Techniques for thyroid FNA: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:407–424. PMID:
18478608.

6. Silverman JF, West RL, Finley JL, Larkin EW, Park HK, Swanson MS, et al. Fine-needle aspiration versus large-needle biopsy or cutting biopsy in evaluation of thyroid nodules. Diagn Cytopathol. 1986; 2:25–30. PMID:
3720480.

7. Wang C, Vickery AL Jr, Maloof F. Needle biopsy of the thyroid. Surg Gynecol Obstet. 1976; 143:365–368. PMID:
959956.
8. Novoa E, Gurtler N, Arnoux A, Kraft M. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: a meta-analysis and systematic review of the literature. Head Neck. 2012; 34:1497–1503. PMID:
22127851.

9. Na DG, Baek JH, Jung SL, Kim JH, Sung JY, Kim KS, et al. Core needle biopsy of the thyroid: 2016 Consensus Statement and Recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017; 18:217–237. PMID:
28096731.

10. Jung CK, Min HS, Park HJ, Song DE, Kim JH, Park SY, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med. 2015; 49:288–299. PMID:
26081825.

11. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update. Endocr Pract. 2016; 22:622–639. PMID:
27167915.
12. Yeon JS, Baek JH, Lim HK, Ha EJ, Kim JK, Song DE, et al. Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology. 2013; 268:274–280. PMID:
23525204.

13. Suh CH, Baek JH, Kim KW, Sung TY, Kim TY, Song DE, et al. The role of core-needle biopsy for thyroid nodules with initially nondiagnostic fine-needle aspiration results: a systematic review and meta-analysis. Endocr Pract. 2016; 22:679–688. PMID:
27176143.

14. Choi SH, Baek JH, Lee JH, Choi YJ, Hong MJ, Song DE, et al. Thyroid nodules with initially non-diagnostic, fine-needle aspiration results: comparison of core-needle biopsy and repeated fine-needle aspiration. Eur Radiol. 2014; 24:2819–2826. PMID:
25038860.

15. Na DG, Kim JH, Sung JY, Baek JH, Jung KC, Lee H, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid. 2012; 22:468–475. PMID:
22304417.

16. Choi YJ, Baek JH, Suh CH, Shim WH, Jeong B, Kim JK, et al. Core-needle biopsy versus repeat fine-needle aspiration for thyroid nodules initially read as atypia/follicular lesion of undetermined significance. Head Neck. 2017; 39:361–369. PMID:
27704650.

17. Park KT, Ahn SH, Mo JH, Park YJ, Park DJ, Choi SI, et al. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck. 2011; 33:160–165. PMID:
20848434.

18. Suh CH, Baek JH, Lee JH, Choi YJ, Kim KW, Lee J, et al. The role of core-needle biopsy in the diagnosis of thyroid malignancy in 4580 patients with 4746 thyroid nodules: a systematic review and meta-analysis. Endocrine. 2016; 54:315–328. PMID:
27220941.

19. Paja M, del Cura JL, Zabala R, Corta I, Lizarraga A, Oleaga A, et al. Ultrasound-guided core-needle biopsy in thyroid nodules. A study of 676 consecutive cases with surgical correlation. Eur Radiol. 2016; 26:1–8. PMID:
25956937.

20. Sung JY, Na DG, Kim KS, Yoo H, Lee H, Kim JH, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol. 2012; 22:1564–1572. PMID:
22415411.

21. Suh CH, Baek JH, Lee JH, Choi YJ, Kim JK, Sung TY, et al. The role of core-needle biopsy as a first-line diagnostic tool for initially detected thyroid nodules. Thyroid. 2016; 26:395–403. PMID:
26651390.

22. Ha EJ, Baek JH, Lee JH, Kim JK, Song DE, Kim WB, et al. Core needle biopsy could reduce diagnostic surgery in patients with anaplastic thyroid cancer or thyroid lymphoma. Eur Radiol. 2016; 26:1031–1036. PMID:
26201291.

23. Ha EJ, Baek JH, Na DG, Kim JH, Kim JK, Min HS, et al. The role of core needle biopsy and its impact on surgical management in patients with medullary thyroid cancer: clinical experience at 3 medical institutions. AJNR Am J Neuroradiol. 2015; 36:1512–1517. PMID:
25929882.

24. Lee SH, Park GS, Jung SL, Kim MH, Bae JS, Lim DJ, et al. Core-needle biopsy for the preoperative diagnosis of follicular neoplasm in thyroid nodule screening: a validation study. Pathol Res Pract. 2016; 212:44–50.

25. Ha EJ, Baek JH, Lee JH, Lee HY, Song DE, Kim JK, et al. A focal marked hypoechogenicity within an isoechoic thyroid nodule: is it a focal malignancy or not? Acta Radiol. 2015; 56:814–819. PMID:
24938659.

26. Ha EJ, Baek JH, Lee JH, Song DE, Kim JK, Shong YK, et al. Sonographically suspicious thyroid nodules with initially benign cytologic results: the role of a core needle biopsy. Thyroid. 2013; 23:703–708. PMID:
23544697.

27. Ha EJ, Baek JH, Lee JH, Kim JK, Kim JK, Lim HK, et al. Core needle biopsy can minimise the non-diagnostic results and need for diagnostic surgery in patients with calcified thyroid nodules. Eur Radiol. 2014; 24:1403–1409. PMID:
24604217.

28. Trimboli P, Nasrollah N, Guidobaldi L, Taccogna S, Cicciarella Modica DD, Amendola S, et al. The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol. 2014; 12:61. PMID:
24661377.

29. Baloch ZW, Cibas ES, Clark DP, Layfield LJ, Ljung BM, Pitman MB, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal. 2008; 5:6. PMID:
18394201.

30. Ha EJ, Baek JH, Lee JH, Kim JK, Choi YJ, Sung TY, et al. Complications following US-guided core-needle biopsy for thyroid lesions: a retrospective study of 6,169 consecutive patients with 6,687 thyroid nodules. Eur Radiol. 2017; 27:1186–1194. PMID:
27311538.

31. Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015; 16:749–766. PMID:
26175574.

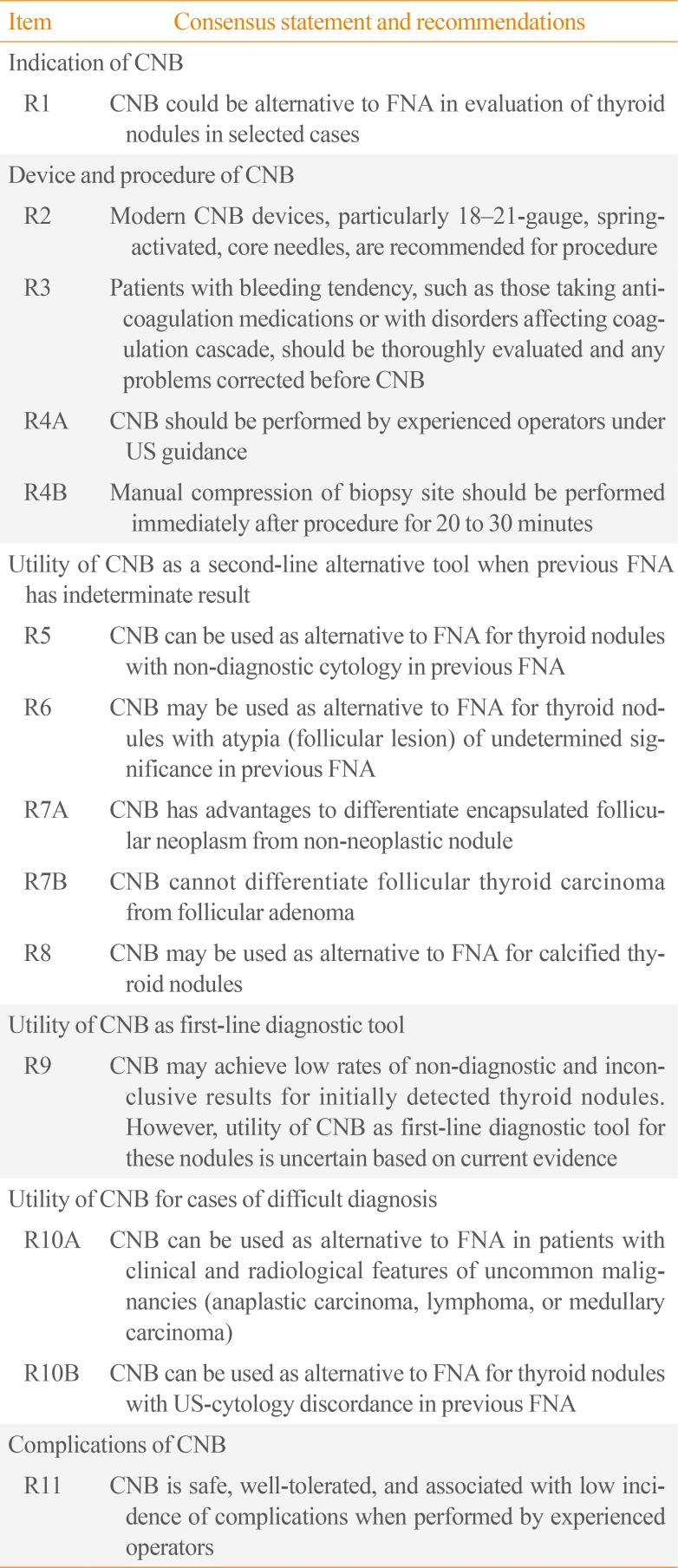
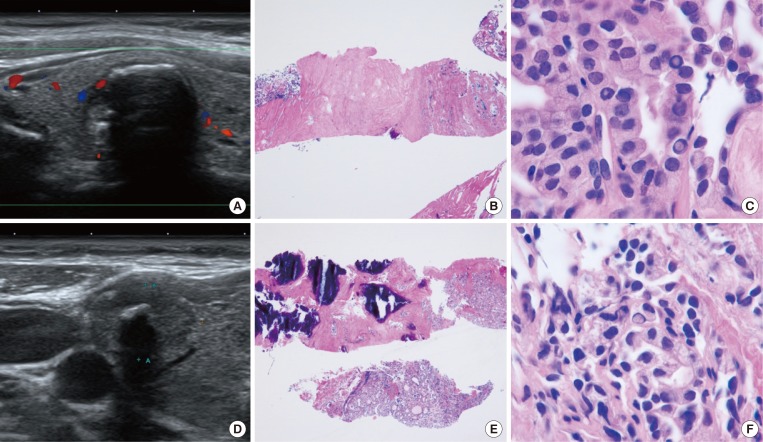
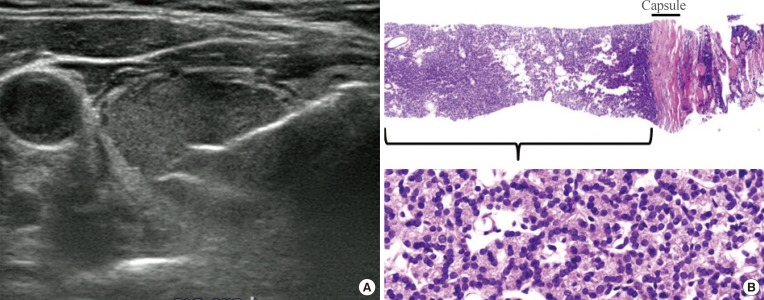
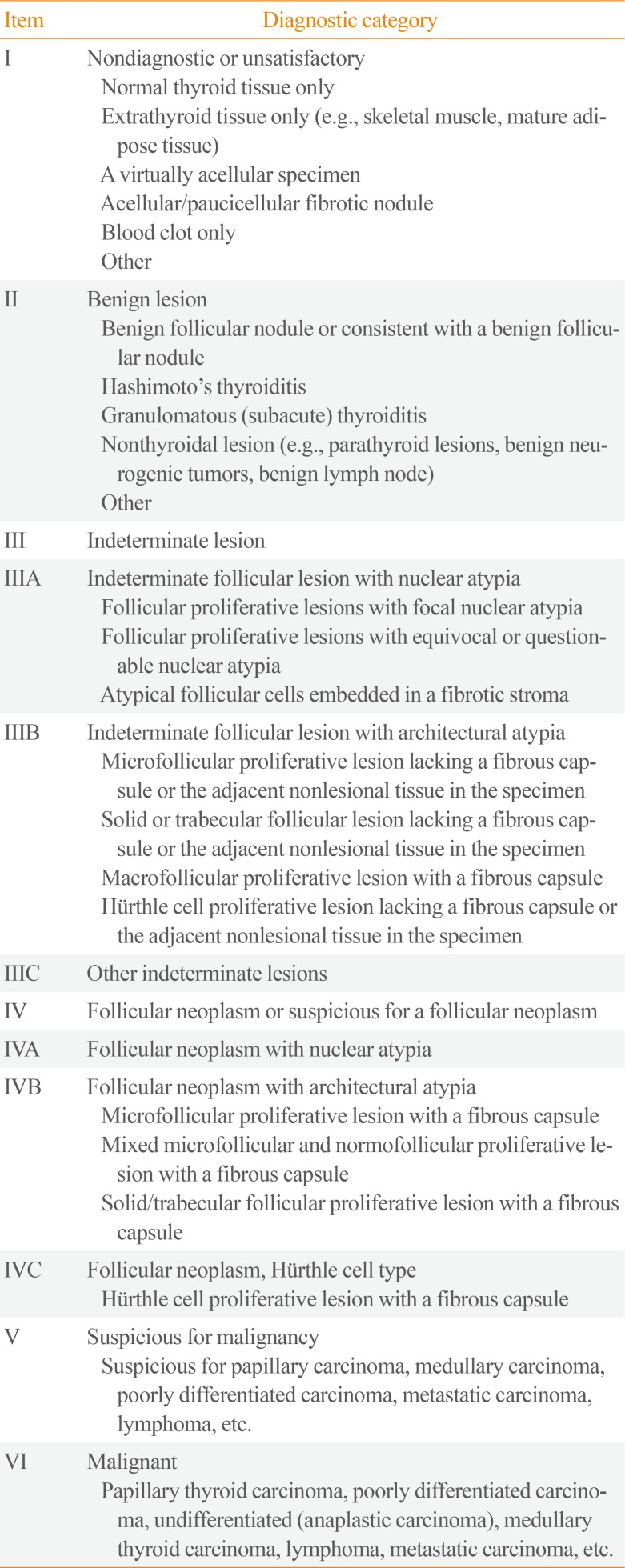




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download