Abstract
Background
Korea is considered an iodine sufficient country, and several studies have been conducted regarding iodine status in healthy Korean adults, pregnant women, and preschool children. However, data on iodine status in Korean school-age children are lacking. Therefore, the iodine nutrition status of Korean school-age children was investigated by measuring urine iodine concentration (UIC).
Methods
This cross-sectional study conducted between April and September 2016 comprised 373 school-age children. UIC was determined using a modified microplate method employing ammonium persulfate digestion followed by Sandell-Kolthoff reaction.
Results
The median UIC was 458.2 µg/L. Excessive iodine intake (>300 µg/L) was found in 286 children (76.7%), with extremely high values exceeding 1,000 µg/L in 19.6% of subjects. Insufficient iodine intake (<100 µg/L) was observed in eight children (2.1%). UIC values were not significantly different between sexes.
Iodine is an essential element needed for the synthesis of thyroid hormones [1]. Both iodine deficiency and excess may lead to thyroid dysfunction or disease [2]. More than 90% of dietary iodine is eventually excreted in the urine, therefore, median urine iodine concentration (UIC) is a good marker of recent dietary iodine intake [3]. World Health Organization (WHO) recommends administering iodine status surveys to school children 6 to 12 years of age due to their vulnerability and easy access [4]. In Korea, several studies have examined iodine status in healthy preschool children, adults, and pregnant women [567]. However, data regarding iodine status of school-age children in Korea are lacking. Therefore, in the present study, the iodine nutrition status of Korean school-age children was investigated by measuring UIC.
The present study included 373 school-age children 7 to 12 years of age from three elementary schools in Busan, the southern coastal region of South Korea. The number of students in the three schools was 290 (A school), 69 (B school), and 24 (C school). Data were collected from April to September 2016. A total of 373 subjects (182 males, 48.8%; 191 females, 51.2%) provided samples for urinary iodine analysis. Height (in cm) and weight (in kg) were measured for all participants. The urine samples were divided into two groups. Groups I and II comprised samples from subjects 7 to 9 and 10 to 12 years of age, respectively.
The study protocol was approved by the Institutional Review Board of Kosin University Gospel Hospital (IRB No: 2014-10-133). Informed written consent was obtained from the children's parents.
A teacher at each school collected midstream voided urine samples (approximately 20 mL) 2 hours after a meal. After breakfast at home, urine samples were collected at school. The collected samples were stored at 4℃ until transferred to the laboratory. UIC was determined using a modified microplate method employing ammonium persulfate digestion followed by the Sandell-Kolthoff reaction [89], a common method used by participants of Ensuring the Quality of Iodine Procedures (EQUIP) of the US Centers for Disease Control and Prevention (CDC) [10]. Briefly, all urine samples and standards (0, 20, 40, 80, 120, 200, and 300 µg/L KIO3) were allowed to reach ambient temperature; then, 250 µL of each sample and standard were mixed with 1 mL of ammonium persulfate solution. The mixtures were incubated on a heating block for 60 minutes at 95℃ and cooled to room temperature. After transferring 50 µL of each mixture to a 96-well plate in duplicate, 100 µL of arsenious acid solution and 50 µL ceric ammonium sulfate solution were added sequentially and incubated for 30 seconds and 30 minutes, respectively. After reading the absorbance at 405 nm, UIC was determined using standard curves constructed for each plate. We evaluated the iodine status according to WHO's epidemiological criteria based on the median UIC of school-age children (Table 1) [4].
Values are expressed as the mean±standard deviation (SD). Because the UIC levels did not show a normal distribution, data were analyzed using the Mann-Whitney and Kruskall-Wallis tests with the SPSS version 23.0 (IBM Co., Armonk, NY, USA). P<0.05 were considered statistically significant.
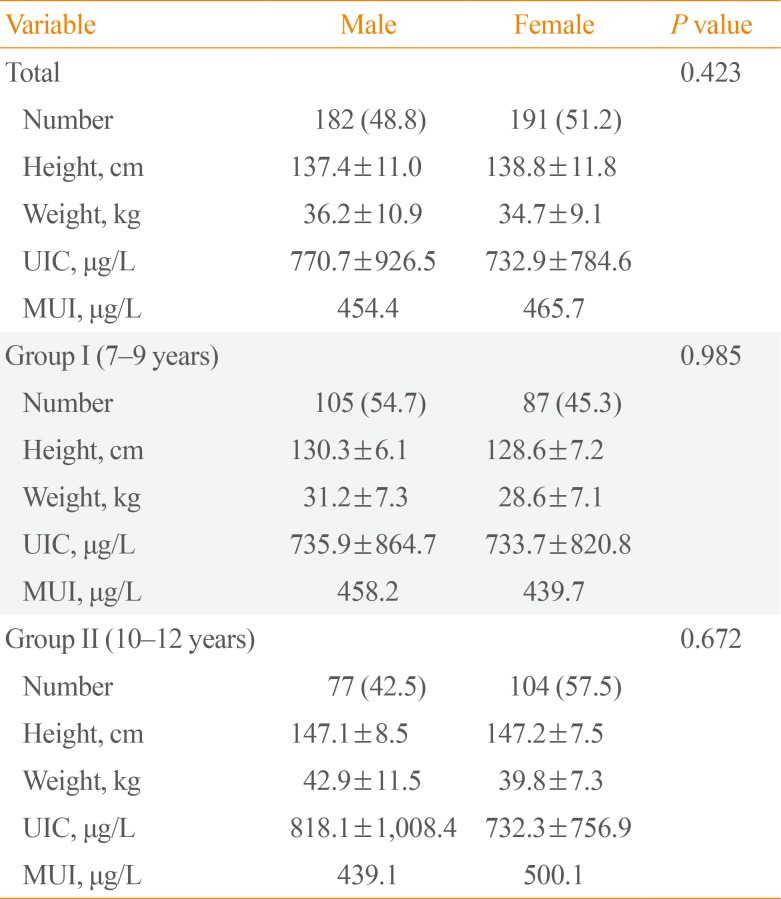
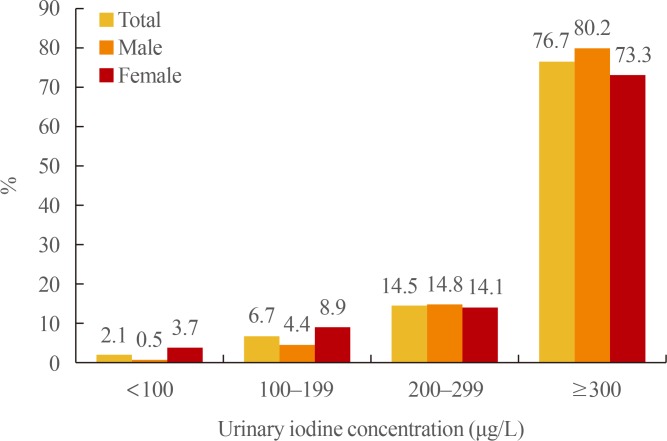
The median UIC was 458.2 µg/L (Table 2). The median UICs for males and females were 454.4 and 465.7 µg/L, respectively. There were no significant differences in UIC between the sexes (P=0.423) (Table 3). Excessive iodine intake (>300 µg/L) was found in 286 children (76.7%), and extremely high values exceeding 1,000 µg/L were found in 73 children (19.6%). Insufficient iodine intake (<100 µg/L) was observed in eight children (2.1%) (Fig. 1).
In the present study, the median UIC in school-age children in Busan, Korea, was 458.2 µg/L. Based on the WHO's epidemiological criteria, the results showed excessive iodine nutrition status of school-age children in Korea., The current global UIC data represent 97.7% of the world population of school-age children [11]. The median UIC of school-age children has been used as the proxy biological indicator of iodine nutrition status in the general population [4]. When data for this age group were not available, data of the next closest age group were used in the following order of priority: data from the children closest to school age, adults, the general population, preschool-age children, and other population groups [4]. Several studies of iodine status in Korea have revealed that the iodine nutritional state of Koreans is more than adequate [12]. In a study of 611 healthy Korean preschool children (2 to 7 years of age), the median UIC was 438.8 µg/L [5]. Another study including 540 healthy adults living in an urban area in Korea found that the median UIC was 268 µg/L [6]. Recently, Cho et al. [7] reported excessive iodine status in 344 Korean healthy pregnant women, reporting a median UIC of 427.3 µg/L and creatinine (Cr)-corrected concentration of 447.9 µg/gCr.
In 2013, 111 countries had adequate iodine nutrition, 30 countries remained iodine deficient, nine that were moderately deficient and 21 that were mildly deficient, and none were considered severely iodine deficient. Only 10 countries showed excessive iodine intake [11]. The results from the present study showed that 76.7% of school-age children had values above >300 µg/L, 35.4% had values >600 µg/L, and 19.6% had extremely high values exceeding 1,000 µg/L. According to Lee et al. [5], 66.4% of Korean preschool children had UIC values >300 µg/L, and 20.9% had values exceeding 1,000 µg/L. In Japan, the median UIC is 281.6 µg/L, and values exceeding 1,000 µg/L were found in 16% of school-age children [4]. Another study reported that the median UIC in children living in coastal Hokkaido was as high as 728 µg/L [13]. People in Japan and Korea generally have much higher iodine intake due to their consumption of substantial amounts of seaweed [614]. Although most individuals tolerate high dietary intakes of iodine, excessive iodine intake can alter thyroid function. Furthermore, many studies have shown that prolonged intake of excessive iodine can be harmful [141516]. Excessive iodine intake in children in high iodine areas is associated with impaired thyroid function and autoimmune thyroid disease [1718]. Li et al. [19] reported that thyroid hypofunction and endemic goiter can develop in children with UIC >800 µg/L. However, Sang et al. [17] observed no difference in the prevalence of subclinical hypothyroidism in children with UIC >800 µg/L compared with those having UIC <800 µg/L based on univariate analysis. They also observed that UIC >600 µg/L and positive anti-thyroperoxidase antibody or anti-thyroglobulin antibody in children were risk factors for subclinical hypothyroidism.
In the present study, most school-age children were iodine sufficient; however, 2.1% of the subjects showed UIC <100 µg/L, indicating iodine deficiency. A similar tendency was observed in a previous study involving Korean preschool children. Lee et al. [5] reported that 3.9% of the subjects showed UIC <100 µg/L. It is unclear why iodine levels in those children were insufficient.
Konig et al. [20] suggested median thresholds for median urinary iodine sufficiency from spot samples identified for study populations, but these should not be applied to individuals because of significant day-to-day variation in salt intake, the main source of dietary iodine in many countries. However, regular screening of UIC might be helpful in preventing iodine deficiency status.
A limitation of this study is that UIC just reflect the immediate intake of food. It could not represent the casual dietary intake. Because the contents of breakfast at home are different, the students' urinary iodine can be very different. Nevertheless, it is meaningful to report that most students are in iodine sufficiency status.
In conclusion, this is the first report of iodine status in school-age children in Korea. The median UIC was 458.2 µg/L, an iodine excess status according to WHO criteria. Although WHO made a guideline for optimal dietary intake of iodine, it is not clear whether UIC over 300 µg/L impacts on thyroid health especially in school ages, since we did not observe any endemics of thyroid disease in Korea or Japan. Therefore further studies are needed to validate this relationship between dietary iodine excess and thyroid disease in different age groups.
ACKNOWLEDGMENTS
This study was supported in part by a grant from Kosin University College of Medicine (to Y.S.C.), and also by Korea National Research Foundation (KNRF) grants 2015M3A9B6073646 and 2015R1D1A1A01058387 (both to J.Y.J.). We are also grateful to elementary schools participated in this study, especially to Principal Sin-Lyeon Lee of Sang-Hak Elementary School for her help in initiating the project.
References
2. Du Y, Gao Y, Meng F, Liu S, Fan Z, Wu J, et al. Iodine deficiency and excess coexist in china and induce thyroid dysfunction and disease: a cross-sectional study. PLoS One. 2014; 9:e111937. PMID: 25375854.

3. Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr. 2012; 142:744–750. PMID: 22378324.

4. International Council for Control of Iodine Deficiency Disorders. UNICEF. World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed. Geneva: World Health Organization; 2007.
5. Lee J, Kim JH, Lee SY, Lee JH. Iodine status in Korean preschool children as determined by urinary iodine excretion. Eur J Nutr. 2014; 53:683–688. PMID: 23881585.

6. Kim JY, Moon SJ, Kim KR, Sohn CY, Oh JJ. Dietary iodine intake and urinary iodine excretion in normal Korean adults. Yonsei Med J. 1998; 39:355–362. PMID: 9752802.

7. Cho YY, Kim HJ, Oh SY, Choi SJ, Lee SY, Joung JY, et al. Iodine status in healthy pregnant women in Korea: a first report. Eur J Nutr. 2016; 55:469–475. PMID: 25750059.

8. Sandell EB, Kolthoff IM. Micro determination of iodine by a catalytic method. Microchimica Acta. 1937; 1:9–25.

9. Wuethrich C, Jaeggi-Groisman SE, Gerber H. Comparison of two methods for the detection of urinary iodine used in epidemiological studies. Clin Chem Lab Med. 2000; 38:1027–1031. PMID: 11140618.

10. Centers for Disease Control and Prevention. Ensuring the quality of urinary iodine procedures. Atlanta: Centers for Disease Control and Prevention;2011.
11. Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid. 2013; 23:523–528. PMID: 23472655.

12. Chung HR. Iodine and thyroid function. Ann Pediatr Endocrinol Metab. 2014; 19:8–12. PMID: 24926457.

13. Zava TT, Zava DT. Assessment of Japanese iodine intake based on seaweed consumption in Japan: a literature-based analysis. Thyroid Res. 2011; 4:14. PMID: 21975053.

14. Lewinski A, Szybinski Z, Bandurska-Stankiewicz E, Grzywa M, Karwowska A, Kinalska I, et al. Iodine-induced hyperthyroidism: an epidemiological survey several years after institution of iodine prophylaxis in Poland. J Endocrinol Invest. 2003; 26:57–62. PMID: 12762642.
15. Zhao J, Wang P, Shang L, Sullivan KM, van der Haar F, Maberly G. Endemic goiter associated with high iodine intake. Am J Public Health. 2000; 90:1633–1635. PMID: 11030003.

16. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006; 354:2783–2793. PMID: 16807415.

17. Sang Z, Chen W, Shen J, Tan L, Zhao N, Liu H, et al. Long-term exposure to excessive iodine from water is associated with thyroid dysfunction in children. J Nutr. 2013; 143:2038–2043. PMID: 24108132.

18. Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010; 24:13–27. PMID: 20172467.

19. Li M, Liu DR, Qu CY, Zhang PY, Qian QD, Zhang CD, et al. Endemic goitre in central China caused by excessive iodine intake. Lancet. 1987; 2:257–259. PMID: 2886725.
20. Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr. 2011; 141:2049–2054. PMID: 21918061.
Table 1
World Health Organization Epidemiological Criteria for Assessing Iodine Nutrition Based on Median UIC
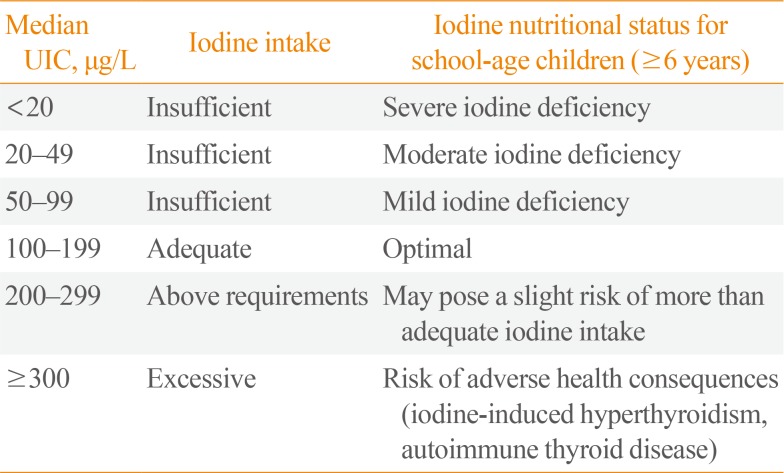
Table 2
Height, Weight, and Median and Mean UIC According to Age
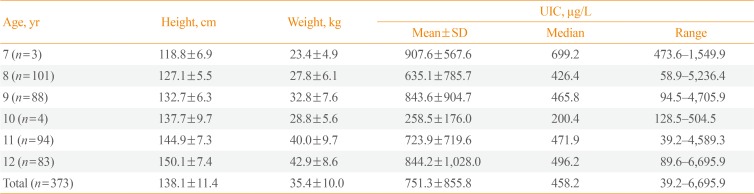
Table 3
Gender Differences in Height, Weight, and UIC According to Age Group




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download