Abstract
In recent studies, the reported prevalence of heterozygous familial hypercholesterolemia (FH) has been higher than in previous reports. Although cascade genetic screening is a good option for efficient identification of affected patients, diagnosis using only clinical criteria is more common in real clinical practice. Cardiovascular risk is much higher in FH patients due to longstanding low density lipoprotein cholesterol (LDL-C) burden and is also influenced by other risk factors. Although guidelines emphasize aggressive LDL-C reduction, the majority of patients cannot reach the LDL-C goal by conventional pharmacotherapy. Novel therapeutics such as proprotein convertase subtilisin/kexin type 9 inhibitors have shown strong lipid lowering efficacy and are expected to improve treatment results in FH patients.
Familial hypercholesterolemia (FH) is a common autosomal dominant genetic disorder and is an important health issue [12]. Patients with FH are exposed to a lifetime burden of high low density lipoprotein cholesterol (LDL-C) and have significantly increased cardiovascular risk. Therefore, early and appropriate diagnosis in affected persons and prevention of vascular complications are critical. In this review, recent studies on the prevalence, diagnosis, vascular risk, and treatment of patient with FH are discussed. Particularly, the focus is on studies of heterozygous FH.
The frequency of heterozygous FH has long been reported to be 1/500 (0.2%), while that of homozygous FH has been reported to be 1/1,000,000 in many studies conducted in various countries around the world [3]. However, although heterozygous FH is influenced by diagnostic criteria including mutation positivity, the prevalence has been reported to be 1/217 in a very recent Danish study [4] and 1/250 in an American study using Dutch criteria [5].
Clinical criteria most commonly used for diagnosis of FH include the Simon Broome Register Group criteria [6], Dutch Lipid Clinic Network criteria [6], and Make Early Diagnosis to Prevent Early Deaths (MEDPED) criteria [7]. A Japanese group has also developed their own criteria for FH [8]. Major items for these clinical diagnostic criteria are (1) severe hypercholesterolemia; (2) physical findings such as xanthoma in the index case; and (3) family history of premature coronary artery disease (CAD) or severe hypercholesterolemia. While the above-mentioned criteria designed in different countries show similarity, they also show difference with respect to specific values (Table 1). For cost-effective and efficient diagnosis, many guidelines and expert groups recommend cascade genetic screening for family members. However, in real practice, diagnosis using only clinical findings from history taking, physical examination, and lipid profile is very common, especially when genetic screening is not available [1].
Most monogenic causes of FH are mutations of low density lipoprotein receptor (LDLR), apolipoprotein B (APOB), or proprotein convertase subtilisin/kexin type 9 (PCSK9) genes. All of these genes are involved in the pathway that clears circulating low-density lipoprotein [9]. Among monogenic FHs, about 90% are caused by mutations of LDLR, for which ≥1,200 different mutations have been identified [1]. However, even family members who have the same mutation often have quite different LDL-C levels, responsiveness to lipid lowering therapy, and survival. Therefore, decision of whether to treat or the intensity of treatment depends mostly on the phenotype rather than genotype. In other words, the clinical importance of genetic diagnosis is limited, and clinical diagnosis is emphasized in real clinical practice [10]. Pathogenic mutations are not identified in about 60% of clinically diagnosed FH patients, although the proportion differs according to the ethnicity or diagnostic criteria. Most FH patients without a monogenic cause are suspected to have polygenic FH that is caused by a multiple lipid-related common variants [2].
Although LDL-C >500 mg/dL or a xanthoma in a child is suspicious for homozygous FH, some patients with homozygous FH have lower LDL-C levels [11].
The most critical complication in FH is life-time cardiovascular disease due to longstanding LDL-C burden (Fig. 1). It has been reported that 25% of affected women and 50% of affected men experience cardiovascular complications [12]. The risk of CAD is known to be 3.5- to 16-fold higher in heterozygous FH patients compared to non-FH individuals [13]. The results of studies on the risk of cerebrovascular disease have been inconsistent and the risk is not yet clear [14]. The guidelines recommend that risk assessment in patients with FH should not be made according to European Systematic Coronary Risk Estimation or American Framingham Risk Score, because these scoring systems underestimate cardiovascular risk in FH [1]. Even with the same LDL-C levels, carriers of mutations for FH show higher risk for CAD [15]. In addition, cardiovascular risk in FH is also influenced by other traditional risk factors, and these often also need to be considered when determining treatment [1416].
The main purpose of preventive measures in FH is the reduction of cardiovascular risk. Thus, it is essential to implement pharmaco- and non-pharmacotherapy targeting hypercholesterolemia and other combined risk factors as well. As mentioned above, because vascular risk is very high and associated with cholesterol burden, lowering LDL-C is the mainstay of cardiovascular prevention in FH. Most recent guidelines indicate that it is desirable to reduce LDL-C to 50% of baseline levels or <100 mg/dL in adults with FH. Statins are the first-line agent and ezetimibe or cholesterol-binding resins are recommended when needed [17]. Data from the United Kingdom have demonstrated that the mortality of patients with FH decreased by 25% to 48% after introduction of statins [18]. Earlier treatment is important for efficient prevention of vascular complications. However, because the evidence is limited, there is no sufficient consensus on when to start treatment in children with FH [192021].
Lipoprotein apheresis can be considered in patients with persistently high LDL-C after treatment, extremely high cardiovascular risk, statin intolerance, or especially in patients who have homozygous FH [1]. Meanwhile, the achievement rate of LDL-C goal (<100 mg/dL) is reported to be 11% [14] and a large part of patients do not reach the target. Therefore, PCSK9 inhibitors, recently approved lipid-lowering agents, are spotlighted and expected to meet the unmet medical need (Table 2) [2223]. Although two PCSK9 inhibitors, evolocumab and alirocumab, have shown consistent and strong lipid-lowering efficacy, limitation is their high cost [24]. Lomitapide inhibits microsomal triglyceride transfer protein and reduces very low density lipoprotein (VLDL) assembly, whereas mipomersen blocks the translation of apoB and decreases VLDL synthesis. These two agents were approved for the treatment of homozygous FH. However, drug-related adverse events are relatively frequent and risk/benefit ratio needs to be taken into account for these drugs (Table 2) [2223].
Although Asian data on FH are not sufficient, its reported prevalence is similar to that of Western countries. The average cholesterol level in Asian patients with FH is likely lower [25]. A recent Korean study suggested that the best cutoff values for predicting mutation-positive FH are total cholesterol 310 mg/dL and LDL-C 225 mg/dL [26]. In Asian patients, 80% to 100% of causing mutations were identified in LDLR, whereas the others were found in APOB or PCSK9 [2526]. Three of 10 Korean FH patients revealed history of CAD. However, the achievement rate of LDL-C by conventional lipid-lowering therapy was not satisfactory [27].
FH is a relatively frequent genetic disorder. Diagnosis of FH by clinical rather than genetic criteria is more common in real world practice. Reduction of premature complications is most critical in patients with FH. Although, the majority of patients cannot reach a LDL-C goal by conventional pharmacotherapy, novel therapeutics such as PCSK9 inhibitors are expected to improve treatment results.
ACKNOWLEDGMENTS
This research was financially supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2012R1A4A1029061 and 2014R1A1A2056104), the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (2015M3A9B602 9138), and the National Research Council of Science & Technology (NST) grant by the Korean government (MSIP) (No. CAP-12-2-KBSI).
References
1. Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013; 34:3478–3900a. PMID: 23956253.
2. Talmud PJ, Shah S, Whittall R, Futema M, Howard P, Cooper JA, et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: a case-control study. Lancet. 2013; 381:1293–1301. PMID: 23433573.

3. Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol. 2004; 160:407–420. PMID: 15321837.

4. Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J. 2016; 37:1384–1394. PMID: 26908947.

5. de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation. 2016; 133:1067–1072. PMID: 26976914.

6. Marks D, Thorogood M, Neil HA, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003; 168:1–14. PMID: 12732381.

7. Hovingh GK, Davidson MH, Kastelein JJ, O'Connor AM. Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J. 2013; 34:962–971. PMID: 23416791.

8. Harada-Shiba M, Arai H, Oikawa S, Ohta T, Okada T, Okamura T, et al. Guidelines for the management of familial hypercholesterolemia. J Atheroscler Thromb. 2012; 19:1043–1060. PMID: 23095242.

9. Soutar AK, Naoumova RP. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Cardiovasc Med. 2007; 4:214–225. PMID: 17380167.

10. Stein EA, Raal FJ. Polygenic familial hypercholesterolaemia: does it matter? Lancet. 2013; 381:1255–1257. PMID: 23433574.

11. Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014; 35:2146–2157. PMID: 25053660.

12. McCrindle BW, Gidding SS. What should be the screening strategy for familial hypercholesterolemia? N Engl J Med. 2016; 375:1685–1686. PMID: 27783919.

13. Hovingh GK, Kastelein JJ. Diagnosis and management of individuals with heterozygous familial hypercholesterolemia: too late and too little. Circulation. 2016; 134:710–712. PMID: 27601557.
14. Perez de Isla L, Alonso R, Mata N, Saltijeral A, Muniz O, Rubio-Marin P, et al. Coronary heart disease, peripheral arterial disease, and stroke in familial hypercholesterolaemia: insights from the SAFEHEART registry (Spanish Familial Hypercholesterolaemia Cohort Study). Arterioscler Thromb Vasc Biol. 2016; 36:2004–2010. PMID: 27444203.
15. Khera AV, Won HH, Peloso GM, Lawson KS, Bartz TM, Deng X, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016; 67:2578–2589. PMID: 27050191.

16. Watts GF, Sullivan DR, Poplawski N, van Bockxmeer F, Hamilton-Craig I, Clifton PM, et al. Familial hypercholesterolaemia: a model of care for Australasia. Atheroscler Suppl. 2011; 12:221–263. PMID: 21917530.

17. Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015; 132:2167–2192. PMID: 26510694.
18. Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J. 2008; 29:2625–2633. PMID: 18840879.

19. Vuorio A, Docherty KF, Humphries SE, Kuoppala J, Kovanen PT. Statin treatment of children with familial hypercholesterolemia: trying to balance incomplete evidence of long-term safety and clinical accountability: are we approaching a consensus? Atherosclerosis. 2013; 226:315–320. PMID: 23141908.
20. Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015; 36:2425–2437. PMID: 26009596.

21. Lozano P, Henrikson NB, Dunn J, Morrison CC, Nguyen M, Blasi PR, et al. Lipid screening in childhood and adolescence for detection of familial hypercholesterolemia: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016; 316:645–655. PMID: 27532919.
22. Ajufo E, Rader DJ. Recent advances in the pharmacological management of hypercholesterolaemia. Lancet Diabetes Endocrinol. 2016; 4:436–446. PMID: 27012540.

23. Santos RD, Gidding SS, Hegele RA, Cuchel MA, Barter PJ, Watts GF, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016; 4:850–861. PMID: 27246162.

24. Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016; 316:743–753. PMID: 27533159.

25. Zhou M, Zhao D. Familial hypercholesterolemia in Asian populations. J Atheroscler Thromb. 2016; 23:539–549. PMID: 27075771.

26. Shin DG, Han SM, Kim DI, Rhee MY, Lee BK, Ahn YK, et al. Clinical features of familial hypercholesterolemia in Korea: predictors of pathogenic mutations and coronary artery disease. A study supported by the Korean Society of Lipidology and Atherosclerosis. Atherosclerosis. 2015; 243:53–58. PMID: 26343872.
27. Lee SH. Characteristics and vascular complications of familial hypercholesterolemia in Korea. J Atheroscler Thromb. 2016; 23:532–538. PMID: 26947601.

Fig. 1
Low density lipoprotein cholesterol (LDL-C) burden in individuals with or without familial hypercholesterolemia (FH) as function of the onset of statin therapy. Adapted from Nordestgaard et al. [7], with permission from Oxford University Press. CHD, coronary heart disease; HDL-C, high density lipoprotein cholesterol.
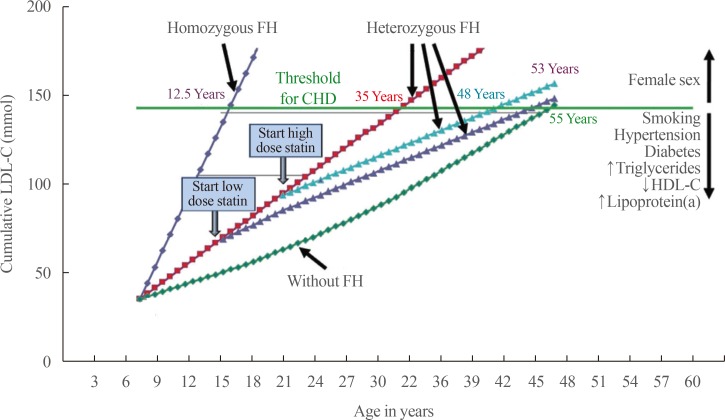
Table 1
Characteristics of Four Clinical Diagnostic Criteria
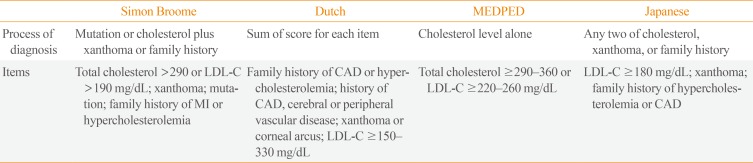
Table 2
New Therapeutics Approved for Familial Hypercholesterolemia
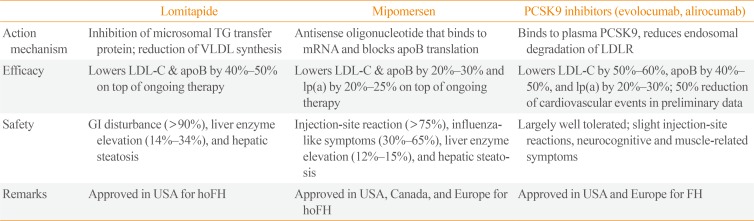
PCSK9, proprotein convertase subtilisin/kexin type 9; TG, triglyceride; VLDL, very low density lipoprotein; apoB, apolipoprotein B; LDLR, low density lipoprotein receptor; LDL-C, low density lipoprotein cholesterol; lp(a), lipoprotein(a); GI, gastrointestinal; hoFH, homozygous familial hypercholesterolemia; FH, familial hypercholesterolemia.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download