Abstract
Discoveries of somatic mutations permit the recognition of subtypes of aldosterone-producing adenomas (APAs) with distinct clinical presentations and pathological features. Catenin β1 (CTNNB1) mutation in APAs has been recently described and discussed in the literature. However, significant knowledge gaps still remain regarding the prevalence, clinical characteristics, pathophysiology, and outcomes in APA patients harboring CTNNB1 mutations. Aberrant activation of the Wnt/β-catenin signaling pathway will further modulate tumorigenesis. We also discuss the recent knowledge of CTNNB1 mutation in adrenal adenomas.
Primary aldosteronism is the most common cause of secondary hypertension with a prevalence of 5% to 10% in hypertensive patients and 20% in patients with treatment-resistant hypertension [1234]. In aldosterone-producing adenoma (APA), the majority of somatic mutations were potassium voltage-gated channel subfamily J member 5 (KCNJ5) mutations (ranging from 52.9% to 76.8% in Asia) [567]. Recently, the prevalence of a novel catenin β1 (CTNNB1) mutation in APAs was 3.7% to 5.1% [58]. We integrate the studies of APAs and show the prevalence of reported somatic mutation in APAs (Fig. 1) [678910111213141516171819202122]. CTNNB1 mutations were associated with stabilized β-catenin and increased AXIN2 (axis inhibition protein 2) expression, suggesting the activation of Wnt signaling [23]. In APA, CTNNB1 mutations occurred mutually exclusively from KCNJ5, ATPase Na+/K+ transporting subunit α1 (ATP1A1), ATPase plasma membrane Ca2+ transporting 3 (ATP2B3), and calcium voltage-gated channel subunit α1 D (CACNA1D) mutated tumors, implying that aberrant Wnt activation plays a pivotal role in APA formation [24]. Accordingly, tumors with CTNNB1 mutations were associated with relatively large adenomas and predominantly expressed in females [8].
The Wnt signaling pathway, through β-catenin signaling, is important for the normal development and maintenance of the adrenal cortex, and more specifically, the zona glomerulosa (ZG) within the cortex [2526]. Somatic mutations of CTNNB1 have been found in 27% of adrenocortical adenomas and 31% of adrenocortical carcinomas [27]. Exon 3 of the CTNNB1 gene (encoding β-catenin) contains specific serine and threonine residues, where phosphorylation marks β-catenin for degradation [28]. Mutations or deletions of exon 3, leading to the aberrant activation of Wnt signaling, subsequently inhibits the phosphorylation of β-catenin [29]. Due to alterations in exon 3 of the CTNNB1 gene, mice with activating Wnt signaling develop hyperaldosteronism and adrenocortical tumors [24]. Mutations in CTNNB1 also cause increased and abnormal Wnt activation in human adrenocortical tumors [2730], and augment the Wnt signaling pathway, leading to tumor formation [31]. In one recent series, cases of APAs harboring activating mutations of β-catenin were described in three women (two during pregnancy and one postmenopausal), who had a heterozygous somatic mutation (C→G, p.Ser33Cys in case 1, C→T, p.Ser45Phe in case 2, and G→A, p.Gly34Arg in case 3) in exon 3 of CTNNB1. All three mutations are predicted to affect a GSK-3β (glycogen synthase kinase 3β) phosphorylation consensus motif and could thus impair β-catenin degradation and up-regulate Wnt activity, resulting in elevated levels of active β-catenin [32].
In the peritumoral tissue of APA, important remodeling of the adrenal cortex has also been observed with reduced vascularization, ZG hyperplasia, and increased nodulation that were not correlated with cytochrome P450 family 11 subfamily B member 2 (CYP11B2) expression [33]. A recent study showed that somatic mutations in the KCNJ5, ATP1A1, or CACNA1D genes are not limited to APAs, but are also found in the more frequent multinodular adrenals [34]. However, in a multinodular gland, the mutation was found in only one nodule, showing that mutation and nodule formation are independent processes [35]. These data demonstrate that the processes of nodule formation and aldosterone hypersecretion can be dissociated in pathological adrenals, suggesting a two-hit model for APA formation. The primary hit, consisting of somatic mutation of one of the known genes in about 60% of cases and of other unknown genetic mutation in the remaining patients, can cause aldosterone hypersecretion. The secondary hit would lead to alterations in the normal balance between adrenocortical cell proliferation and apoptosis, triggering nodule formation (Fig. 2) [3637]. Of note, activation of the Wnt/β-catenin pathway further modulates the two hits required for both adrenal nodule formation and increased aldosterone secretion [2338]. APAs harboring CTNNB1 mutation could display CYP11B1 or CYP11B2 heterogeneous expression [8], or in both CYP11B2-positive and CYP11B2-negative regions [39]. It is also consistent with our result that the Wnt/β-catenin pathway activates downstream cyclin D1 transcription, which is a gene involved in cell growth [40] in adenomas with CTNNB1 mutations compared with wild-type APA adenomas. All of these findings, together with the reported higher prevalence of CTNNB1 mutations among other adrenal adenomas [41] and adrenal cancers [23], suggest that CTNNB1 mutations may be more related to tumor cell growth (tumorigenesis), rather than to actual aldosterone production.
Two subgroups of APAs were observed: one with diffuse CYP11B2 expression with concomitantly low CYP11B1 expression, and one with low CYP11B2 and high CYP11B1 expressions [8]. APAs harboring CTNNB1 mutations have shown variable expression of CYP11B1 and CYP11B2 [5832]. Of note, APAs harboring CTNNB1 mutations could express luteinizing hormone/choriogonadotropin receptor (LHCGR) and gonadotropin releasing hormone receptor (GNRHR), encoding gonadal receptors, at levels that were more than 100 times higher than the levels in other APAs in one report [32]. Constitutive activation of the Wnt signaling pathway in ZG-like adenomatous cells could lead to de-differentiation toward the common adrenal-gonadal precursor cell type, and to the aberrant expression of gonadal receptors LHCGR and/or GNRHR [5]. However, GNRHR could present diffuse cytoplasmic, membranous, and nuclear expression in adenomas, and was especially enhanced in adenomas harboring CTNNB1 mutations from female patients. GNRHR was attenuated in KCNJ5 mutated adenomas. LHCGR was diffusely expressed in adrenal tissues and was prominent in adenomas harboring CTNNB1 mutations [5]. Compared with KCNJ5 mutated APAs, no difference in CYP11B1 expression levels were observed, but significantly higher CYP11B2 expression was observed in CTNNB1 mutated tumors in a single report [8].
CTNNB1 mutated APAs were more often observed in female patients (60% to 75%) [58] and older patients, with a shorter duration of hypertension [5]. There were no significant differences in preoperative aldosterone levels, tumor size at surgery, and the ratio of parental hypertension in patients with tumors harboring CTNNB1 mutations compared to those with tumors harboring KCNJ5 mutations [58]. However, CTNNB1 mutations led to higher serum potassium and creatinine levels compared to KCNJ5 mutations in one study [5].
Patients with tumors harboring CTNNB1 mutation have a small but increased risk of malignant transformation [27]. Experiments using β-catenin mutated mice which develop benign tumors can transition to malignancy, indicating the requirement of additional epigenetic changes [2442]. This is consistent with the multistep progression model seen in patients with familial adenomatous polyposis [43]. However, most APAs rarely increase in size and the transition to aldosterone-producing carcinomas is extremely rare [44]. Adrenal carcinomas harboring CTNNB1 mutation are also extremely rare.
According to our study, CTNNB1 mutation carriers had a higher possibility (87.5%) of residual hypertension than other APA patients after adrenalectomy [5]. Compared with KCNJ5 mutation carriers (12.5% vs. 79.3%, P<0.001), CTNNB1 mutation carriers had a much lower chance of recovery from hypertension, even after 1-year follow-up. One of the possible explanations of the higher postadrenalectomy residual hypertension among patients harboring CTNNB1 mutations could be that age-related essential hypertension plays an important role in the hypertension observed in these patients.
CTNNB1 mutations and activation of the Wnt/β-catenin pathway are also found in other benign and malignant adrenocortical neoplasms that do not produce aldosterone, including cortisol producing adenomas (CPA) [454647]. As previously stated, activated Wnt/β-catenin signaling contributes to adrenal tumorigenesis [48]. CTNNB1 mutation has been described in a 4-month-old Thai infant with Cushing's syndrome secondary to bilateral adrenal tumors with hepatic metastasis [49]. Following molecular investigations, a deletion mutation of β-catenin involving codons 44 to 45 was detected in the right adrenal tumor and peripheral blood of this patient, which indicates systemic mutation. Immunohistochemistry showed nuclear accumulation of β-catenin on the right adrenal tumor together with the metastatic nodule in the liver and the left adrenal tumor harbored wild-type β-catenin.
For CPAs, mutations in the catalytic subunit of protein kinase A (PKA) were identified and shown to occur mutually exclusively to CTNNB1 mutations [5051]. The PKA pathway has paramount importance in the regulation of adrenocortical growth and hormone secretion. Activating mutations in PKA led to constitutively activated cyclic adenosine monophosphate (cAMP) signaling, causing increased cortisol production and tumor formation. Expression analysis revealed the increased expression of genes involved in the biosynthesis and metabolism of steroids in tumors with protein kinase cAMP-activated catalytic subunit α (PRKACA) mutation [50]. Somatic gain-of-function mutations in the PRKACA have been found in cortisol-producing adrenocortical adenomas [50515253], but the presence of genetic alterations in genes involved in the PKA pathway in APA is currently unknown. ARMC5 (armadillo repeat containing 5) is a gene found to be mutated in macronodular adrenal hyperplasia and has a connection with the PKA pathway [54].
CTNNB1 mutations in a subset of APAs are predominant with aberrant β-catenin accumulation. Tumors harboring these mutations have a variable histological and CYP11B2/B1 expression pattern, and show different clinical characteristics, such as female gender dominance and a higher risk of postadrenalectomy residual hypertension. CTNNB1 mutations in APAs could relate to tumorigenesis rather than aldosterone production by activating Wnt/β-catenin signaling.
Figures and Tables
 | Fig. 1The prevalence of the most known mutation of aldosterone producing adenoma. This box plot displays the full range of variation (from maximum, mean, medium to minimum, accordingly) in each index somatic mutation. CACNA1D, calcium voltage-gated channel subunit α1 D; KCNJ5, potassium voltage-gated channel subfamily J member 5; ATP2B3, ATPase plasma membrane Ca2+ transporting 3; ATP1A1, ATPase Na+/K+ transporting subunit α1; GNAS, guanine nucleotide binding protein, α stimulating; CTNNB1, catenin β1. |
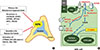 | Fig. 2A two-hit model for the pathogenesis of aldosterone-producing adenoma (APA). (A, B) Primary hit: Somatic mutations in CACNA1D, KCNJ5, ATP2B3, ATP1A1, and possibly other genetic alterations produce cell depolarization, increased cytoplasmic calcium level and increased CYP11B2 expression, causing aldosterone hypersecretion. Secondary hit: Aberrant activation of signaling pathways (such as Wnt/β-catenin, Shh, PKA, etc.) causes imbalances between cell proliferation and death in the adrenal, leading to adenoma formation. (A) Adapted from Lalli et al., with permission from Elsevier [36]. (B) Adapted from Seidel et al. [37]. CACNA1D, calcium voltage-gated channel subunit α1 D; KCNJ5, potassium voltage-gated channel subfamily J member 5; ATP2B3, ATPase plasma membrane Ca2+ transporting 3; ATP1A1, ATPase Na+/K+ transporting subunit α1; APCC, aldosterone-producing cell clusters; HSD3b, hydroxy-δ-5-steroid dehydrogenase, 3β- and steroid δ-isomerase cluster; CTNNB1, catenin β1; DACH1, dachshund family transcription factor 1; Shh, sonic hedgehog; PKA, protein kinase A; Ang II, angiotensin II; AT1, Ang II type 1; CAMK, Ca2+/calmodulin-dependent protein kinase; CYP11B2, cytochrome P450 family 11 subfamily B member 2. |
ACKNOWLEDGMENTS
This research was supported by Chi Mei Medical Center, Liouying (grant number, CLFHR10609), and Ministry of Science and Technology (MOST) of the Republic of China (Taiwan) (grant number, MOST 106-2321-B-182-002). We also express our sincere gratitude to all staff of the Taiwan Clinical Trial Consortium, TCTC.
References
1. Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004; 89:1045–1050.
2. Fogari R, Preti P, Zoppi A, Rinaldi A, Fogari E, Mugellini A. Prevalence of primary aldosteronism among unselected hypertensive patients: a prospective study based on the use of an aldosterone/renin ratio above 25 as a screening test. Hypertens Res. 2007; 30:111–117.
3. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006; 48:2293–2300.
4. Hannemann A, Bidlingmaier M, Friedrich N, Manolopoulou J, Spyroglou A, Volzke H, et al. Screening for primary aldosteronism in hypertensive subjects: results from two German epidemiological studies. Eur J Endocrinol. 2012; 167:7–15.
5. Wu VC, Wang SM, Chueh SJ, Yang SY, Huang KH, Lin YH, et al. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci Rep. 2017; 7:39121.
6. Wu VC, Huang KH, Peng KY, Tsai YC, Wu CH, Wang SM, et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep. 2015; 5:11396.
7. Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. 2015; 65:622–628.
8. Akerstrom T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016; 6:19546.
9. Xekouki P, Hatch MM, Lin L, Rodrigo de A, Azevedo M, de la Luz Sierra M, et al. KCNJ5 mutations in the National Institutes of Health cohort of patients with primary hyperaldosteronism: an infrequent genetic cause of Conn's syndrome. Endocr Relat Cancer. 2012; 19:255–260.
10. Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, et al. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J Clin Endocrinol Metab. 2012; 97:1311–1319.
11. Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb. 2015; 22:191–200.
12. Boulkroun S, Beuschlein F, Rossi GP, Golib-Dzib JF, Fischer E, Amar L, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. 2012; 59:592–598.
13. Azizan EA, Murthy M, Stowasser M, Gordon R, Kowalski B, Xu S, et al. Somatic mutations affecting the selectivity filter of KCNJ5 are frequent in 2 large unselected collections of adrenal aldosteronomas. Hypertension. 2012; 59:587–591.
14. Kuppusamy M, Caroccia B, Stindl J, Bandulik S, Lenzini L, Gioco F, et al. A novel KCNJ5-insT149 somatic mutation close to, but outside, the selectivity filter causes resistant hypertension by loss of selectivity for potassium. J Clin Endocrinol Metab. 2014; 99:E1765–E1773.
15. Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf). 2015; 83:779–789.
16. Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013; 45:1050–1054.
17. Nakajima Y, Okamura T, Horiguchi K, Gohko T, Miyamoto T, Satoh T, et al. GNAS mutations in adrenal aldosterone-producing adenomas. Endocr J. 2016; 63:199–204.
18. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013; 45:440–444.
19. Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension. 2014; 63:188–195.
20. Akerstrom T, Crona J, Delgado Verdugo A, Starker LF, Cupisti K, Willenberg HS, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One. 2012; 7:e41926.
21. Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014; 64:354–361.
22. Hong AR, Kim JH, Song YS, Lee KE, Seo SH, Seong MW, et al. Genetics of aldosterone-producing adenoma in Korean patients. PLoS One. 2016; 11:e0147590.
23. Boulkroun S, Samson-Couterie B, Golib-Dzib JF, Amar L, Plouin PF, Sibony M, et al. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology. 2011; 152:4753–4763.
24. Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010; 19:1561–1576.
25. Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002; 143:4358–4365.
26. Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, et al. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008; 135:2593–2602.
27. Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagnere AM, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005; 65:7622–7627.
28. Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002; 108:837–847.
29. Bjorklund P, Lindberg D, Akerstrom G, Westin G. Stabilizing mutation of CTNNB1/beta-catenin and protein accumulation analyzed in a large series of parathyroid tumors of Swedish patients. Mol Cancer. 2008; 7:53.
30. Durand J, Lampron A, Mazzuco TL, Chapman A, Bourdeau I. Characterization of differential gene expression in adrenocortical tumors harboring beta-catenin (CTNNB1) mutations. J Clin Endocrinol Metab. 2011; 96:E1206–E1211.
31. Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997; 275:1787–1790.
32. Teo AE, Garg S, Shaikh LH, Zhou J, Karet Frankl FE, Gurnell M, et al. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N Engl J Med. 2015; 373:1429–1436.
33. Boulkroun S, Samson-Couterie B, Dzib JF, Lefebvre H, Louiset E, Amar L, et al. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension. 2010; 56:885–892.
34. Fernandes-Rosa FL, Giscos-Douriez I, Amar L, Gomez-Sanchez CE, Meatchi T, Boulkroun S, et al. Different somatic mutations in multinodular adrenals with aldosterone-producing adenoma. Hypertension. 2015; 66:1014–1022.
35. Dekkers T, ter Meer M, Lenders JW, Hermus AR, Schultze Kool L, Langenhuijsen JF, et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab. 2014; 99:E1341–E1351.
36. Lalli E, Barhanin J, Zennaro MC, Warth R. Local control of aldosterone production and primary aldosteronism. Trends Endocrinol Metab. 2016; 27:123–131.
37. Seidel E, Scholl UI. Intracellular molecular differences in aldosterone: compared to cortisol-secreting adrenal cortical adenomas. Front Endocrinol (Lausanne). 2016; 7:75.
38. El Wakil A, Lalli E. The Wnt/beta-catenin pathway in adrenocortical development and cancer. Mol Cell Endocrinol. 2011; 332:32–37.
39. Nanba K, Chen AX, Omata K, Vinco M, Giordano TJ, Else T, et al. Molecular heterogeneity in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016; 101:999–1007.
40. Safe S, Kasiappan R. Natural products as mechanism-based anticancer agents: sp transcription factors as targets. Phytother Res. 2016; 30:1723–1732.
41. Tadjine M, Lampron A, Ouadi L, Bourdeau I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf). 2008; 68:264–270.
42. Heaton JH, Wood MA, Kim AC, Lima LO, Barlaskar FM, Almeida MQ, et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am J Pathol. 2012; 181:1017–1033.
43. Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, Rai K, et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009; 137:623–634.
44. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab. 2016; 101:1889–1916.
45. Bonnet S, Gaujoux S, Launay P, Baudry C, Chokri I, Ragazzon B, et al. Wnt/β-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and non-secreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab. 2011; 96:E419–E426.
46. Gaujoux S, Grabar S, Fassnacht M, Ragazzon B, Launay P, Libe R, et al. β-Catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin Cancer Res. 2011; 17:328–336.
47. Thiel A, Reis AC, Haase M, Goh G, Schott M, Willenberg HS, et al. PRKACA mutations in cortisol-producing adenomas and adrenal hyperplasia: a single-center study of 60 cases. Eur J Endocrinol. 2015; 172:677–685.
48. Berthon A, Drelon C, Ragazzon B, Boulkroun S, Tissier F, Amar L, et al. WNT/β-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet. 2014; 23:889–905.
49. Pusantisampan T, Sangkhathat S, Kayasut K, Kanngurn S, Jaruratanasirikul S, Chotsampancharoen T, et al. Cushing's syndrome in an infant secondary to malignant adrenocortical tumors with somatic mutation of beta-catenin. Pediatr Dev Pathol. 2010; 13:238–242.
50. Cao Y, He M, Gao Z, Peng Y, Li Y, Li L, et al. Activating hotspot L205R mutation in PRKACA and adrenal Cushing's syndrome. Science. 2014; 344:913–917.
51. Beuschlein F, Fassnacht M, Assie G, Calebiro D, Stratakis CA, Osswald A, et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing's syndrome. N Engl J Med. 2014; 370:1019–1028.
52. Goh G, Scholl UI, Healy JM, Choi M, Prasad ML, Nelson-Williams C, et al. Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors. Nat Genet. 2014; 46:613–617.
53. Sato Y, Maekawa S, Ishii R, Sanada M, Morikawa T, Shiraishi Y, et al. Recurrent somatic mutations underlie corticotropin-independent Cushing's syndrome. Science. 2014; 344:917–920.
54. Assie G, Libe R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing's syndrome. N Engl J Med. 2013; 369:2105–2114.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download