This article has been corrected. See "Identification of Maturity-Onset Diabetes of the Young Caused by Glucokinase Mutations Detected Using Whole-Exome Sequencing" in Volume 36 on page 468.
Abstract
Glucokinase maturity-onset diabetes of the young (GCK-MODY) represents a distinct subgroup of MODY that does not require hyperglycemia-lowering treatment and has very few diabetes-related complications. Three patients from two families who presented with clinical signs of GCK-MODY were evaluated. Whole-exome sequencing was performed and the effects of the identified mutations were assessed using bioinformatics tools, such as PolyPhen-2, SIFT, and in silico modeling. We identified two mutations: p.Leu30Pro and p.Ser383Leu. In silico analyses predicted that these mutations result in structural conformational changes, protein destabilization, and thermal instability. Our findings may inform future GCK-MODY diagnosis; furthermore, the two mutations detected in two Korean families with GCK-MODY improve our understanding of the genetic basis of the disease.
Inactivating heterozygous mutations in the glucokinase (GCK) gene cause maturity-onset diabetes of the young (GCK-MODY), also known as MODY2, which was first described in 1992 [1]. Because patients with GCK-MODY have a defect in glucose sensing, glucose homeostasis is maintained at a higher set point; this results in mild, asymptomatic fasting hyperglycemia from birth. GCK-MODY is very different from other MODY subtypes. For example, patients with MODY3, a subtype characterized by hepatocyte nuclear factor 1 homeobox A (HNF1A) mutations, are born with normoglycemia and develop diabetes as adolescents or young adults. In the MODY3 subtype, diabetes progresses, resulting in marked hyperglycemia, with substantial treatment requirements and an increased risk of diabetes-associated complications. In contrast, in individuals with GCK-MODY, diabetes-related micro- and macrovascular complications are rare, and patients usually do not require treatment, except during pregnancy [2]. Patients with GCK-MODY are often initially misdiagnosed as having early type 2 diabetes mellitus (T2DM), type 1 diabetes mellitus (T1DM). Accordingly, genetic testing is essential to avoid unnecessary treatment and investigations.
A GCK-MODY prevalence of 1.1 in 1,000 (95% confidence interval, 0.3 to 2.9) was found in a predominantly white European population [2]. Although MODY has been identified in Asian populations, its prevalence in Korea is not known. Only one patient with GCK-MODY has been reported in studies of the Korean population [34]. The extremely low rate of GCK-MODY identification is attributed to its asymptomatic and benign nature, as well as to misclassification as either T1DM or T2DM or impaired fasting glucose levels. More importantly, the low GCK-MODY identification rate is impacted by the lack of knowledge about this condition among health care professionals and by the cost of genetic testing.
Whole-exome sequencing (WES) is a useful method for discovering rare, unknown germline mutations in Mendelian disorders, such as GCK-MODY [5]. In this study, we conducted WES for three patients from two families with diabetes and suspected GCK-MODY based on their clinical background and medical history. We identified and characterized GCK mutations in these two families.
From February to May 2016, we performed genetic analysis to identify GCK mutations in two families. The clinical and family histories of the patients and fasting glucose and 75-g oral glucose tolerance test (OGTT) results were evaluated. Serum measurements of diabetic autoimmune antibodies were also analyzed.
In June 2012, a 27-year-old woman (proband) was referred to the diabetes clinic because the type of diabetes was uncertain. Elevated fasting hyperglycemia (133 mg/dL) was incidentally detected. Her initial glycated hemoglobin (HbA1c) was 6.4%, her fasting serum C-peptide level was 0.60 ng/mL, and the anti-glutamic acid decarboxylase (GAD) antibody test was negative. The standard 75-g OGTT showed an increase in blood glucose from 115 to 202 mg/dL after 2 hours. Her body mass index (BMI) was 16.7 kg/m2. There were no diabetic family members (Fig. 1A). Despite metformin use (500 mg daily), the fasting blood glucose level was not reduced, and the HbA1c level was stably maintained within 6.1% to 6.4% from June 2012 to March 2014. Moreover, even after a 3-year withdrawal from anti-diabetic medication, her glucose and HbA1c levels were un-changed.
In March 2016, a 21-year-old man (proband) visited the clinic and fasting hyperglycemia (140 mg/dL) was incidentally detected. He did not complain of polyuria, polydipsia, or weight loss, and his BMI was 24.0 kg/m2. His mother and maternal grandmother also had diabetes. A 48-year-old woman, the mother of the proband, was diagnosed with gestational diabetes at age 26, during her pregnancy with the proband. The birth weight of the proband was 3.2 kg, even though the mother did not receive treatment, dietary modifications, or exercise therapy. Her blood glucose did not return to normal after the delivery and her fasting glucose level was persistently high. The maternal grandmother of the proband also had diabetes, although the time of onset is unknown, and she suffered from frequent hypoglycemic symptoms, but only took one oral hypoglycemic agent. One maternal sister and one maternal brother also have diabetes (Fig. 1B). The initial HbA1c of the proband was 6.9%, and his fasting serum C-peptide level was 0.73 ng/mL. The anti-GAD antibody test was negative, and the standard 75-g OGTT showed an in crease in blood glucose from 144 to 241 mg/dL after 2 hours. The BMI of the mother of the proband was 26.9 kg/m2 and HbA1c was 7.0%. The anti-GAD antibody was also undetected. The standard 75-g OGTT showed an increase in blood glucose from 133 to 177 mg/dL after 2 hours.
WES and, when necessary, Sanger sequencing were performed. WES was performed with patient genomic DNA using the SureSelect Human All Exon V5 (Agilent Technologies, Santa Clara, CA, USA). Sequencing was performed using the HiSeq2000 system (Illumina, San Diego, CA, USA). The generated reads were mapped against the UCSC hg19 human genome reference. Sequence variants were compared to those reported in PubMed and in the following public databases (accessed November 2016): HGMD (Human Gene Mutation Database, http://www.hgmd.cf.ac.uk/ac/index.php), LOVD (Leiden Open Variation Database, http://grenada.lumc.nl/LOVD2/diabetes/home.php?select_db=GCK), and dbSNP (Single Nucleotide Polymorphism Database, https://www.ncbi.nlm.nih.gov/SNP/index.html). The structural and/or functional consequences of amino acid substitutions were predicted using the SIFT (http://sift.jcvi.org/), polymorphism phenotyping v2 (PolyPhen-2; http://genet-ics.bwh.harvard.edu/pph2/index.shtml), and DUET (http://bleoberis.bioc.cam.ac.uk/duet/) algorithms [6]. Models for GCK mutants were generated in silico using the active form of the GCK crystal structure as a template (Protein Database code [PDB code] 1V4S).
Genetic testing for the proband by WES revealed a p.L30P missense mutation in the glucokinase gene. Using Sanger sequencing, we confirmed the existence of the mutation (Fig. 2). This missense mutation has been described previously [7], and was found in Italian children with incidental hyperglycemia [8]. The amino acid change at position 30 from leucine to proline, p.L30P, was predicted to lead to destabilization in the protein structure, as modeled using the DUET algorithm (Table 1).
Genetic testing of the proband by WES revealed a p.S383L missense mutation in GCK. WES of the mother of the proband revealed the same p.S383L missense mutation in GCK. Based on an in silico analysis, the p.S383L mutation was predicted to cause structural conformational changes, potentially leading to protein destabilization and a loss of protein function (Table 1). This mutation has also been reported in one Spanish family with GCK-MODY [9].
An accurate diagnostic approach for patients with GCK-MODY is important to avoid unnecessary, expensive analyses and the misclassification of the type of diabetes. In this study, we characterized mutations in the GCK gene using WES and bioinformatics methods in two families with suspected GCK-MODY. In Korea, very few studies have focused on the discovery of patients with MODY [4]. Shim et al. [10] did not find any causative alleles of known MODY 1–13 genes by WES in six Korean MODY probands and their family members. Therefore, it was believed that there is a major discrepancy in the genetic basis of the disease between Korean and Caucasian patients, despite a lack of definitive evidence. However, the mutations identified in this study have been reported previously in Italian and Spanish families [79]. Accordingly, we inferred that the low rate of discovery of patients with MODY in Korea can likely be attributed to the lack of knowledge among physicians and the high cost of genetic testing.
In this study, the precise selection of patients who presented the typical phenotype of GCK-MODY enabled us to find potential causal mutations, despite the lack of diabetic family members for the proband in family 1. Usually, MODY patients have a strong family history of diabetes of any type, insulin independence, an absence of autoantibodies for pancreatic antigens, and evidence of endogenous insulin production. The diagnosis of MODY is based on specific criteria, e.g., age, BMI, number of affected generations, presence of diabetes symptoms, and geographical origin [11]. However, using traditional criteria, around half of all MODY cases are not properly diagnosed [12]. Subjects with GCK-MODY have a range of clinical presentations. It can present at any age from birth, whereas HNF1A-MODY can present into the fifth decade of life. The number of affected generations may not be clear because patients are generally asymptomatic, and hyperglycemia is commonly discovered during routine screening, pregnancy, or insurance medicals. Almost all patients inherit the GCK mutation from an affected parent; however, the parents may not be diagnosed with diabetes or mild hyperglycemia, and accordingly there may not be a known family history [2]. The clinical presentation of GCK-MODY is usually incidental. Clinical suspicion is essential for the diagnosis of GCK-MODY when a strong family history is lacking.
A diagnosis of GCK-MODY can only be confirmed by molecular genetic testing to identify a heterozygous mutation in GCK. A previously described WES technique [13] together with targeted resequencing [14] is useful for discovering unknown or rare germline mutations in Mendelian disorders, such as monogenic diabetes. Following the identification of GCK mutations using these methods, understanding the functional consequences of these mutations (i.e., the phenotypic effect) is a major challenge for clinicians. Inactivating mutations in GCK result in a decrease in phosphorylation potential, which results in a reduction in β-cell glucose usage. However, a limited number of mutations have been functionally characterized [15]. Recently, to overcome the laborious and time-consuming nature of the experimental analysis of all GCK mutations, several researchers have developed evolution and structure-based computational strategies [16]. Several studies using both experimental and computational methods have demonstrated the structural and functional effects of missense mutations in GCK. These techniques can be used to differentiate disease-related and neutral mutations [17]. In this study, we also evaluated the effects of two mutations using bioinformatics tools, i.e., PolyPhen-2, SIFT, DUET, and in silico modeling. Both mutations were predicted to be deleterious and displayed thermal instability.
Patients with GCK-MODY are often initially misdiagnosed with early T1DM or T2DM diabetes. Because treatment, follow-up, and prognosis are different from those for the more common forms of MODY, the correct identification of GCK-MODY is clinically important. Even after 50 years of mild hyperglycemia, people with GCK-MODY do not develop significant microvascular complications, and the prevalence of macrovascular complications is probably similar to that in the general population [18]. Treatment is not recommended outside of pregnancy because glucose lowering therapy is ineffective in people with GCK-MODY and long-term complications are rare [2]. Extensive reviews encompassing the clinical phenotype, presentation, and differential diagnosis of GCK-MODY can be found in the literature [215].
This study had several limitations. First, the parents in family 1 might carry the same missense mutation as the proband. Unfortunately, we did not receive consent from the parents for genetic analyses. We cannot definitively determine whether the p.L30P missense mutation is de novo or not. Secondly, although targeted resequencing is more sensitive and accurate than WES for detecting monogenic diabetes, we could not use this method owing to the high cost and unavailability of service vendors. Thirdly, we were not able to analyze mutations in other family members of each proband. In this respect, this study is incomplete. Nonetheless, our results demonstrate that if the clinical phenotype is well-defined, clinical suspicion can lead to a diagnosis of GCK-MODY. In the United Kingdom, it is estimated that >80% of MODY cases are misdiagnosed as T1DM or T2DM [12]. For individuals with MODY to receive the most appropriate treatment, an active screening strategy is essential. In Norway, Johansson et al. [19] performed a screening study for MODY in all antibody-negative children in a nationwide population-based registry. They reported that the prevalence of MODY in antibody-negative children with diabetes might be as high as 6.5%. We hope that many physicians who treat diabetes consider GCK-MODY and attempt to identify more patients.
In conclusion, we identified two mutations in two Korean families with GCK-MODY using WES and predicted their phenotypic and functional effects using bioinformatics analyses.
Figures and Tables
Fig. 1
(A, B) Pedigrees of families harboring glucokinase (GCK) missense variants. Filled and empty symbols represent diabetic and non-diabetic individuals, respectively. Arrows indicate probands.

Fig. 2
Confirmation of the heterozygote missense mutation (c.92T>C, p.Leu30Pro) identified in the proband of family 1 by Sanger sequencing.
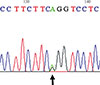
Table 1
Bioinformatics Analysis of GCK Mutations

Two mutations were predicted to be deleterious using online prediction tools. DUET is a web server that uses an integrated computational approach to study missense mutations in proteins; it is available at http://structure.bioc.cam.ac.uk/duet.
GCK, glucokinase; PolyPhen-2, polymorphism phenotyping v2.
ACKNOWLEDGMENTS
This study was supported by research fund of Kangwon Branch of Korean Endocrine Society (2017).
References
1. Froguel P, Vaxillaire M, Sun F, Velho G, Zouali H, Butel MO, et al. Close linkage of glucokinase locus on chromosome 7p to early-onset non-insulin-dependent diabetes mellitus. Nature. 1992; 356:162–164.
2. Chakera AJ, Steele AM, Gloyn AL, Shepherd MH, Shields B, Ellard S, et al. Recognition and management of individuals with hyperglycemia because of a heterozygous glucokinase mutation. Diabetes Care. 2015; 38:1383–1392.
3. Hwang JS, Shin CH, Yang SW, Jung SY, Huh N. Genetic and clinical characteristics of Korean maturity-onset diabetes of the young (MODY) patients. Diabetes Res Clin Pract. 2006; 74:75–81.
4. Kim SH. Maturity-onset diabetes of the young: what do clinicians need to know? Diabetes Metab J. 2015; 39:468–477.
5. Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011; 12:745–755.
6. Pires DE, Ascher DB, Blundell TL. DUET: a server for predicting effects of mutations on protein stability using an integrated computational approach. Nucleic Acids Res. 2014; 42(Web Server issue):W314–W319.
7. Gloyn AL. Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum Mutat. 2003; 22:353–362.
8. Lorini R, Klersy C, d'Annunzio G, Massa O, Minuto N, Iafusco D, et al. Maturity-onset diabetes of the young in children with incidental hyperglycemia: a multicenter Italian study of 172 families. Diabetes Care. 2009; 32:1864–1866.
9. Barrio R, Bellanne-Chantelot C, Moreno JC, Morel V, Calle H, Alonso M, et al. Nine novel mutations in maturity-onset diabetes of the young (MODY) candidate genes in 22 Spanish families. J Clin Endocrinol Metab. 2002; 87:2532–2539.
10. Shim YJ, Kim JE, Hwang SK, Choi BS, Choi BH, Cho EM, et al. Identification of candidate gene variants in Korean MODY families by whole-exome sequencing. Horm Res Paediatr. 2015; 83:242–251.
11. Bellanne-Chantelot C, Levy DJ, Carette C, Saint-Martin C, Riveline JP, Larger E, et al. Clinical characteristics and diagnostic criteria of maturity-onset diabetes of the young (MODY) due to molecular anomalies of the HNF1A gene. J Clin Endocrinol Metab. 2011; 96:E1346–E1351.
12. Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010; 53:2504–2508.
13. Teer JK, Mullikin JC. Exome sequencing: the sweet spot before whole genomes. Hum Mol Genet. 2010; 19:R145–R151.
14. Ellard S, Lango Allen H, De Franco E, Flanagan SE, Hysenaj G, Colclough K, et al. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013; 56:1958–1963.
15. Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanne-Chantelot C, Ellard S, et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009; 30:1512–1526.
16. George DC, Chakraborty C, Haneef SA, Nagasundaram N, Chen L, Zhu H. Evolution- and structure-based computational strategy reveals the impact of deleterious missense mutations on MODY 2 (maturity-onset diabetes of the young, type 2). Theranostics. 2014; 4:366–385.
17. Capuano M, Garcia-Herrero CM, Tinto N, Carluccio C, Capobianco V, Coto I, et al. Glucokinase (GCK) mutations and their characterization in MODY2 children of southern Italy. PLoS One. 2012; 7:e38906.
18. Steele AM, Shields BM, Wensley KJ, Colclough K, Ellard S, Hattersley AT. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA. 2014; 311:279–286.
19. Johansson BB, Irgens HU, Molnes J, Sztromwasser P, Aukrust I, Juliusson PB, et al. Targeted next-generation sequencing reveals MODY in up to 6.5% of antibody-negative diabetes cases listed in the Norwegian Childhood Diabetes Registry. Diabetologia. 2017; 60:625–635.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download