Abstract
Background
No previous studies have been published on poorly differentiated thyroid carcinoma (PDTC) in Southeast Asia.
Methods
We included all adult PDTC patients diagnosed using the Turin criteria at the Philippine General Hospital from 2006 to 2015. The data collected included demographics, clinical presentation, histopathology, treatment, and outcomes. Tests of association were employed to compare these data with foreign studies on PDTC, as well as with local studies on well differentiated thyroid carcinoma (WDTC) and anaplastic thyroid carcinoma (ATC).
Results
Eighteen PDTC cases were identified. The median age was 62 years old, with the majority being females. All patients had goiter on presentation, and most were stage IV at the time of diagnosis. In terms of PDTC subtype, insular and trabecular patterns were equally common. Extrathyroidal extension was documented in eight patients, while five patients each had nodal and distant metastasis. All but one patient underwent surgery; however, less than half received adjuvant radioiodine therapy. The 5-year survival rate was 83%. Three patients (16.7%) died at a median of 12 months after diagnosis. Nine (50%) are still alive with persistent and/or recurrent disease at a median of 39 months after diagnosis.
Conclusion
The behavior of PDTC in this Southeast Asian population was found to be similar to patterns observed in other regions, and exhibited intermediate features between WDTC and ATC. Appropriate surgery provided excellent 5-year survival rates, but the role of adjuvant therapy remains unclear. Larger studies are needed to identify prognostic factors in this population.
Thyroid malignancies are predicted to become the fifth leading cause of cancer in women worldwide [1]. They are categorized along a continuum, with well differentiated thyroid carcinoma (WDTC) on one end and anaplastic thyroid carcinoma (ATC) on the other. Poorly differentiated thyroid carcinoma (PDTC) exhibits a unique histologic architecture and represents the bridge between WDTC and ATC [2]. It was previously classified as a WDTC variant until being recognized in 2004 by the World Health Organization Classification of Endocrine Tumors as a distinct entity, defined as a “follicular cell neoplasm with limited evidence of structural follicular cell differentiation that occupies morphologically and behaviorally an intermediate position between differentiated and undifferentiated carcinoma” [3]. This definition was further refined in the 2006 Turin criteria, which stated that a thyroid malignancy must demonstrate a trabecular, insular, or solid (TIS) growth pattern; the absence of the conventional nuclear patterns of papillary carcinoma; and the presence of at least one of the following features: convoluted nuclei, mitotic activity, or necrosis [4].
Similarly to other thyroid cancer subtypes, PDTC occurs more commonly in women, most often in the sixth decade of life [56]. It is generally rare, with an incidence rate of 0.23% to 2.6% among patients with thyroid carcinomas [78]. Regional variations exist, with frequencies of up to 15% in Northern Italy, suggesting that risk factors such as iodine deficiency and radiation exposure play a role in disease development [9]. Consistent with their intermediate differentiation, PDTCs can display both local and distant aggressiveness. The poor prognostic factors identified in the literature include age >45 years, a mitotic index of >3/high power field (HPF), necrosis, a tumor size >4 cm, higher stage, positive surgical margins, local recurrence, and distant metastasis [81011].
Because of its rarity and intermittent biologic aggressiveness, knowledge regarding the optimal management of PDTC remains limited. Furthermore, the majority of published data are from Western countries; data from Southeast Asia are lacking. Given the ethnic and geographic differences in tumor behavior, this study aimed to fill this knowledge gap by documenting the 10-year experience of a tertiary medical center in the Philippines with regard to PDTC.
The study protocol was approved by the Institutional Technical and Ethics Review Board prior to its commencement.
All patients >18 years old diagnosed with a thyroid malignancy from 2006 to 2015 at the Philippine General Hospital (PGH) were identified using logbooks from the Department of Pathology. For purposes of standardization, only cases with histopathology performed at the PGH Department of Pathology were included. The slides were reviewed by a pathologist to determine whether a diagnosis of PDTC was made according to the Turin criteria. For confirmed PDTC cases, patient records were retrieved and a data collection form was used to collect information regarding demographics, clinical and histopathological characteristics, the treatment given, and the clinical course and outcome. Those with incomplete data and/or who were lost to follow-up were included and reported accordingly. Patient data were tabulated and compared with data from foreign studies on PDTC, as well as with data on WDTC and ATC patients in the Philippines. In our institution, there is no single standard protocol for the treatment of PDTC.
Statistical analysis was performed using Stata SE version 13 software (StataCorp, College Station, TX, USA). Descriptive statistics included the mean±standard deviation for normally distributed quantitative variables, the median and interquartile range for non-normally distributed quantitative variables, and the frequency and percentage for qualitative variables. Tests of association (the t test for independent means and the Z test for two proportions) were employed to compare these characteristics with PDTC patients from other countries, as well as with WDTC and ATC patients in the Philippines. Statistical significance was set at P<0.05.
Out of the 761 thyroid malignancies identified over the 10-year period, a total of 18 cases satisfied the Turin criteria for the diagnosis of PDTC. The median age was 62 years (range, 40 to 75), with females comprising the majority (72%). There was no documented history of radiation exposure in any patient. All patients had goiter on presentation, with a mean size of 8.6 cm and a median duration of 9 years. More than half (61%) were TNM (tumor, node, metastasis) stage IV on diagnosis, with a mean maximum tumor dimension of 5.8 cm and a mean mitotic index of 10.1/HPF. Necrosis was present in the majority (72%). In terms of PDTC subtype, insular and trabecular patterns were equally common. Extrathyroidal extension was documented in eight patients, while five patients each had nodal and distant metastasis. With regard to treatment, all but one patient underwent surgery, and more than half underwent at least a total thyroidectomy with neck dissection as necessary. In contrast, less than half underwent adjuvant radioactive iodine (RAI) ablation, and no patients received external beam radiotherapy (EBRT) or chemotherapy. Prior to being diagnosed with PDTC, four patients were diagnosed with WDTC and one patient had Hashimoto thyroiditis. The 5-year survival rate was 83%. Three patients (16.7%) died at a median of 12 months after diagnosis. For cases of recurrence, the median time from diagnosis to recurrence was 39 months. Table 1 summarizes the patient characteristics, which are individually shown in Table 2. Fig. 1, on the other hand, shows the survival curves of the study patients after PDTC diagnosis.
This is the first study to document the characteristics of PDTC patients in a Southeast Asian population. We compared our data with those of PDTC patients from other regions (Table 3) and found similarities in terms of age and female predominance. The median age and mean tumor size of our sample, however, were the highest across all studies. These, coupled with the long duration of goiter, can be explained by a general delay in diagnosis due to limitations in medical access experienced by many patients treated at our institution.
The rates of extrathyroidal extension, lymph node metastasis, and distant metastasis in our study are also consistent with the findings of other studies. In general, extrathyroidal extension has been observed in 30% to 59% of PDTC patients, while distant metastasis has been found to occur in 13% to 33% of cases. The exceptions are the case series of Cherkaoui et al. [8] in Morocco, which did not find any patient with extrathyroidal extension or distant metastasis (as all cases were TNM stage III or lower); the study of Volante et al. [6] in Italy, which did not report nodal or distant metastasis, but reported 54% of cases to exhibit wide vascular invasion (>3 blood vessels outside the tumor capsule); and the study of Lin et al. [12] in Taiwan, which did not report extrathyroidal extension or nodal metastasis, but documented eight patients with local invasion. These findings are consistent with the inherent biologic aggressiveness of this disease.
It is important to note that aside from our study, only one other PDTC study utilized the Turin criteria in case selection [8]. The rest utilized a combination of criteria including TIS growth patterns coupled with vascular invasion or follicular differentiation with tumor necrosis [5671112]. Such differences in selection criteria may have influenced the survival rate. For instance, while most studies reported 5-year survival rates of >80%, the studies of Jung et al. [11] in Korea, Lin et al. [12] in Taiwan, and Ibrahimpasic et al. [7] in the USA reported figures of only >60%. A common feature of the Korean and American studies is the presence of necrosis—a known poor prognostic factor—as a prerequisite for case inclusion [711]. In contrast, the Taiwan study had the highest rate of distant metastasis, which may have accounted for the lower survival rate [12]. For studies that utilized the Turin or TIS criteria for diagnosis, there appeared to be no predominant subtype; our study found insular and trabecular patterns to be equally common, while other studies had discrepant findings.
Given the lack of previous standard diagnostic criteria, there are no universal guidelines on the management of PDTC patients. The initial treatment commonly consists of total thyroidectomy with neck dissection whenever necessary [13]. The role of adjuvant therapies such as RAI, EBRT, and chemotherapy are less clear, as no study has shown these treatments to have definite benefits [7]. Thus, they are often reserved for patients with a high locoregional recurrence risk, those with gross residual or inoperable disease, and those needing palliative therapy [14]. Our study had the lowest RAI rate across all studies. This may reflect the financial constraints of many patients in our institution, as well as the reluctance of healthcare providers to pursue a treatment modality less established for this indication. Despite the low RAI rate, however, our study still found a 5-year survival rate of 83%. New therapies in the form of mo-lecular inhibitors targeting the TP53 gene, the MAP (mitogen-activated protein) kinase pathway, and/or the PI3K (phosphoinositide 3-kinase)/AKT pathway may offer additional options to high-risk PDTC patients [15].
We also compared our study results to data on Filipino WDTC patients (Table 4). While the same female predominance was found as was observed among Filipino PDTC patients, the WDTC patients were significantly younger, had smaller tumor sizes on presentation, and had higher rates of stage I disease and lower rates of stage IV disease and distant metastasis. In contrast, the rates of nodal metastasis did not appear to differ between these two groups. This can be explained by the fact that the majority of WDTC cases involved papillary thyroid carcinoma, which exhibits a tendency for nodal metastasis [16]. In terms of treatment, significantly more WDTC patients received curative surgery (at least a total thyroidectomy), while significantly more PDTC patients received only palliative surgery (tumor debulking with or without tracheostomy), as the greater tumor burden of the latter group often precluded complete surgical resection. This may have contributed to the presence of a significantly lower 5-year survival rate and higher 5-year persistence and/or recurrence rates in PDTC patients than in WDTC patients, consistent with the literature [171819]. Finally, the fact that three PDTC cases had prior WDTC supports the theory that thyroid cancer can behave as a continuum with regard to the progression of genetic alterations [13].
We also compared our study results to data on Filipino ATC patients (Table 5). Filipino ATC patients were observed to have significantly larger tumors and higher rates of nodal metastasis and obstructive symptoms. The 5-year survival rate was also significantly lower for Filipino ATC patients, with a significantly shorter duration from diagnosis to death [20]. In all, our study results are generally consistent with the finding that the behavior of PDTC is intermediate between WDTC and ATC.
Our study faced several limitations. A history of prior neck irradiation was not elicited in any patient since neck irradiation as a form of therapy has not been widely used in the Philippines in the past [16]. Iodine status was also not evaluated in any patient, although iodine deficiency has been posited as a risk factor for PDTC development. Genetic and molecular data were also not available. These limitations stemmed from the retrospective design of the study. We also had a very small sample size due to the rarity of the disease and the limited availability of slides (only from the past 10 years) in the Department of Pathology. This precluded the performance of multivariate regression analysis to determine possible prognostic factors in our patients.
In conclusion, the behavior of PDTC in this Southeast Asian population was similar to patterns observed in other regions, with intermediate features between WDTC and ATC. With appropriate surgery, excellent 5-year survival rates can be achieved. The role of adjuvant therapy, however, remains unclear. We recommend larger studies to determine factors predictive of poorer outcomes and mortality in these patients.
References
1. Siegel R, Jemal A. Cancer facts and figures 2012. Atlanta: American Cancer Society;2012.
2. Sadow PM, Faquin WC. Poorly differentiated thyroid carcinoma: an incubating entity. Front Endocrinol (Lausanne). 2012; 3:77. PMID: 22737144.

3. DeLellis RA. World Health Organization. International Agency for Research on Cancer. Chapter 2. Poorly differentiated carcinoma. Pathology and genetics of tumours of endocrine organs. 3rd ed. Lyon: IARC Press;2004. p. 73–76.
4. Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007; 31:1256–1264. PMID: 17667551.

5. Tanaka K, Sonoo H, Saito W, Ohta Y, Shimo T, Sohda M, et al. Analysis of clinical outcome of patients with poorly differentiated thyroid carcinoma. ISRN Endocrinol. 2011; 2011:308029. PMID: 22363874.
6. Volante M, Landolfi S, Chiusa L, Palestini N, Motta M, Codegone A, et al. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer. 2004; 100:950–957. PMID: 14983490.
7. Ibrahimpasic T, Ghossein R, Carlson DL, Nixon I, Palmer FL, Shaha AR, et al. Outcomes in patients with poorly differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2014; 99:1245–1252. PMID: 24512493.

8. Cherkaoui GS, Guensi A, Taleb S, Idir MA, Touil N, Benmoussa R, et al. Poorly differentiated thyroid carcinoma: a retrospective clinicopathological study. Pan Afr Med J. 2015; 21:137. PMID: 26327974.

9. Walczyk A, Kowalska A, Sygut J. The clinical course of poorly differentiated thyroid carcinoma (insular carcinoma): own observations. Endokrynol Pol. 2010; 61:467–473. PMID: 21049460.
10. Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006; 106:1286–1295. PMID: 16470605.
11. Jung TS, Kim TY, Kim KW, Oh YL, Park DJ, Cho BY, et al. Clinical features and prognostic factors for survival in patients with poorly differentiated thyroid carcinoma and comparison to the patients with the aggressive variants of papillary thyroid carcinoma. Endocr J. 2007; 54:265–274. PMID: 17379963.

12. Lin JD, Chao TC, Hsueh C. Clinical characteristics of poorly differentiated thyroid carcinomas compared with those of classical papillary thyroid carcinomas. Clin Endocrinol (Oxf). 2007; 66:224–228. PMID: 17223992.

13. Hannallah J, Rose J, Guerrero MA. Comprehensive literature review: recent advances in diagnosing and managing patients with poorly differentiated thyroid carcinoma. Int J Endocrinol. 2013; 2013:317487. PMID: 23476646.

14. Garcia-Rostan G, Sobrinho-Simoes M. Poorly differentiated thyroid carcinoma: an evolving entity. Diagn Histopathol. 2011; 17:114–123.

15. Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008; 21(Suppl 2):S37–S43. PMID: 18437172.

16. Lo TE, Uy AT, Maningat PD. Well-differentiated thyroid cancer: the Philippine General Hospital experience. Endocrinol Metab (Seoul). 2016; 31:72–79. PMID: 26754584.

17. Sanders EM Jr, LiVolsi VA, Brierley J, Shin J, Randolph GW. An evidence-based review of poorly differentiated thyroid cancer. World J Surg. 2007; 31:934–945. PMID: 17431717.

18. Bongiovanni M, Sadow PM, Faquin WC. Poorly differentiated thyroid carcinoma: a cytologic-histologic review. Adv Anat Pathol. 2009; 16:283–289. PMID: 19700938.
19. Patel KN, Shaha AR. Poorly differentiated and anaplastic thyroid cancer. Cancer Control. 2006; 13:119–128. PMID: 16735986.

20. Lo TE, Jimeno CA, Paz-Pacheco E. Anaplastic thyroid cancer: experience of the Philippine General Hospital. Endocrinol Metab (Seoul). 2015; 30:195–200. PMID: 26194079.

Fig. 1
Survival curves of Filipino patients after poorly differentiated thyroid carcinoma (PDTC) diagnosis.
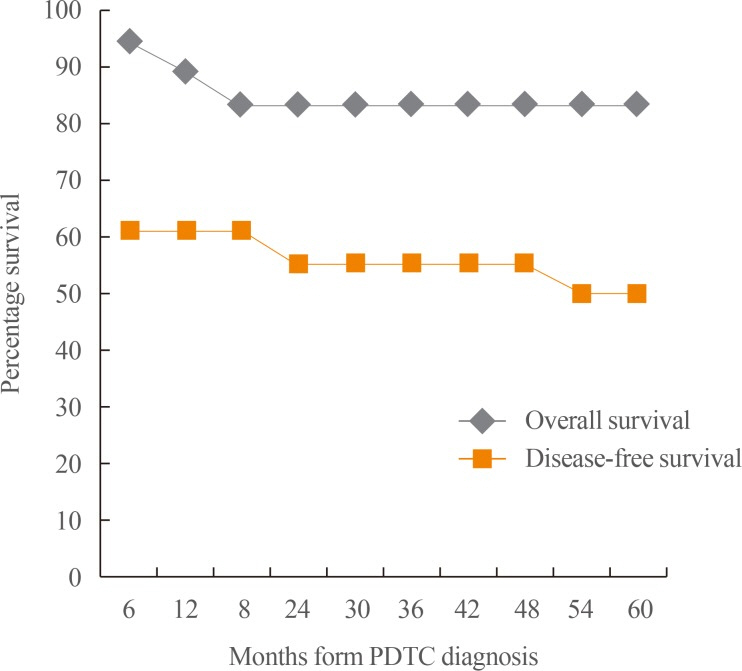
Table 1
Summary Characteristics of Poorly Differentiated Thyroid Carcinoma Cases (n=18)
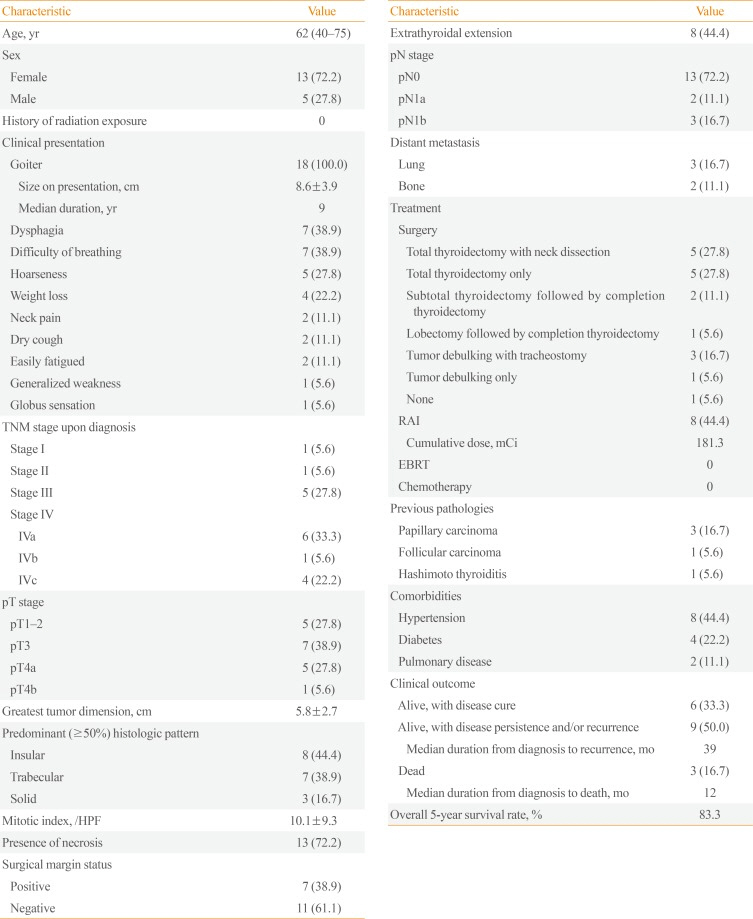
Table 2
Individual Characteristics of Poorly Differentiated Thyroid Carcinoma Cases (n=18)
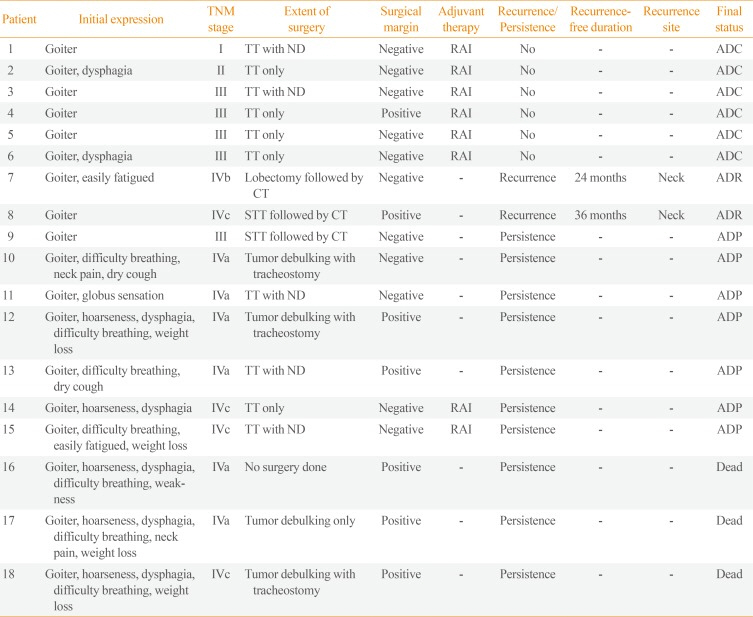
Table 3
Comparison of Features of PDTC Patients among Different Studies
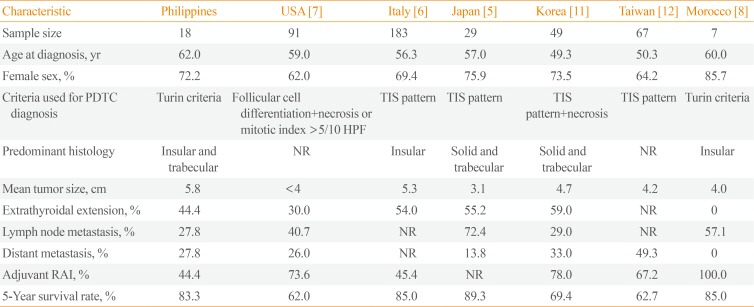
| Characteristic | Philippines | USA [7] | Italy [6] | Japan [5] | Korea [11] | Taiwan [12] | Morocco [8] |
|---|---|---|---|---|---|---|---|
| Sample size | 18 | 91 | 183 | 29 | 49 | 67 | 7 |
| Age at diagnosis, yr | 62.0 | 59.0 | 56.3 | 57.0 | 49.3 | 50.3 | 60.0 |
| Female sex, % | 72.2 | 62.0 | 69.4 | 75.9 | 73.5 | 64.2 | 85.7 |
| Criteria used for PDTC diagnosis | Turin criteria | Follicular cell differentiation+necrosis or mitotic index >5/10 HPF | TIS pattern | TIS pattern | TIS pattern+necrosis | TIS pattern | Turin criteria |
| Predominant histology | Insular and trabecular | NR | Insular | Solid and trabecular | Solid and trabecular | NR | Insular |
| Mean tumor size, cm | 5.8 | <4 | 5.3 | 3.1 | 4.7 | 4.2 | 4.0 |
| Extrathyroidal extension, % | 44.4 | 30.0 | 54.0 | 55.2 | 59.0 | NR | 0 |
| Lymph node metastasis, % | 27.8 | 40.7 | NR | 72.4 | 29.0 | NR | 57.1 |
| Distant metastasis, % | 27.8 | 26.0 | NR | 13.8 | 33.0 | 49.3 | 0 |
| Adjuvant RAI, % | 44.4 | 73.6 | 45.4 | NR | 78.0 | 67.2 | 100.0 |
| 5-Year survival rate, % | 83.3 | 62.0 | 85.0 | 89.3 | 69.4 | 62.7 | 85.0 |
Table 4
Features of Filipino PDTC Patients as Compared to WDTC Patients
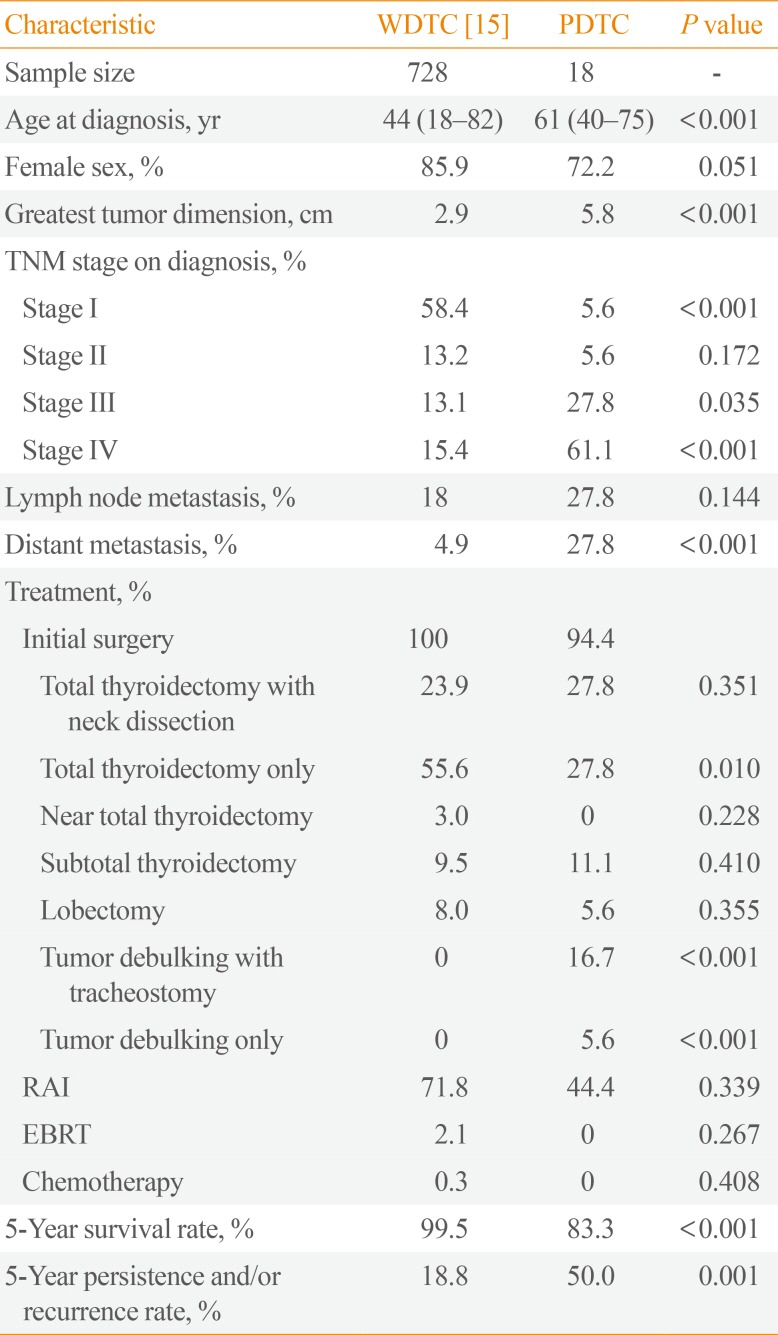
| Characteristic | WDTC [15] | PDTC | P value |
|---|---|---|---|
| Sample size | 0728 | 18 | - |
| Age at diagnosis, yr | 44 (18–82) | 61 (40–75) | <0.001 |
| Female sex, % | 85.9 | 72.2 | 0.051 |
| Greatest tumor dimension, cm | 2.9 | 5.8 | <0.001 |
| TNM stage on diagnosis, % | |||
| Stage I | 58.4 | 5.6 | <0.001 |
| Stage II | 13.2 | 5.6 | 0.172 |
| Stage III | 13.1 | 27.8 | 0.035 |
| Stage IV | 15.4 | 61.1 | <0.001 |
| Lymph node metastasis, % | 18 | 27.8 | 0.144 |
| Distant metastasis, % | 4.9 | 27.8 | <0.001 |
| Treatment, % | |||
| Initial surgery | 100 | 94.4 | |
| Total thyroidectomy with neck dissection | 23.9 | 27.8 | 0.351 |
| Total thyroidectomy only | 55.6 | 27.8 | 0.010 |
| Near total thyroidectomy | 3.0 | 0 | 0.228 |
| Subtotal thyroidectomy | 9.5 | 11.1 | 0.410 |
| Lobectomy | 8.0 | 5.6 | 0.355 |
| Tumor debulking with tracheostomy | 0 | 16.7 | <0.001 |
| Tumor debulking only | 0 | 5.6 | <0.001 |
| RAI | 71.8 | 44.4 | 0.339 |
| EBRT | 2.1 | 0 | 0.267 |
| Chemotherapy | 0.3 | 0 | 0.408 |
| 5-Year survival rate, % | 99.5 | 83.3 | <0.001 |
| 5-Year persistence and/or recurrence rate, % | 18.8 | 50.0 | 0.001 |
Table 5
Features of Filipino PDTC Patients as Compared to ATC Patients
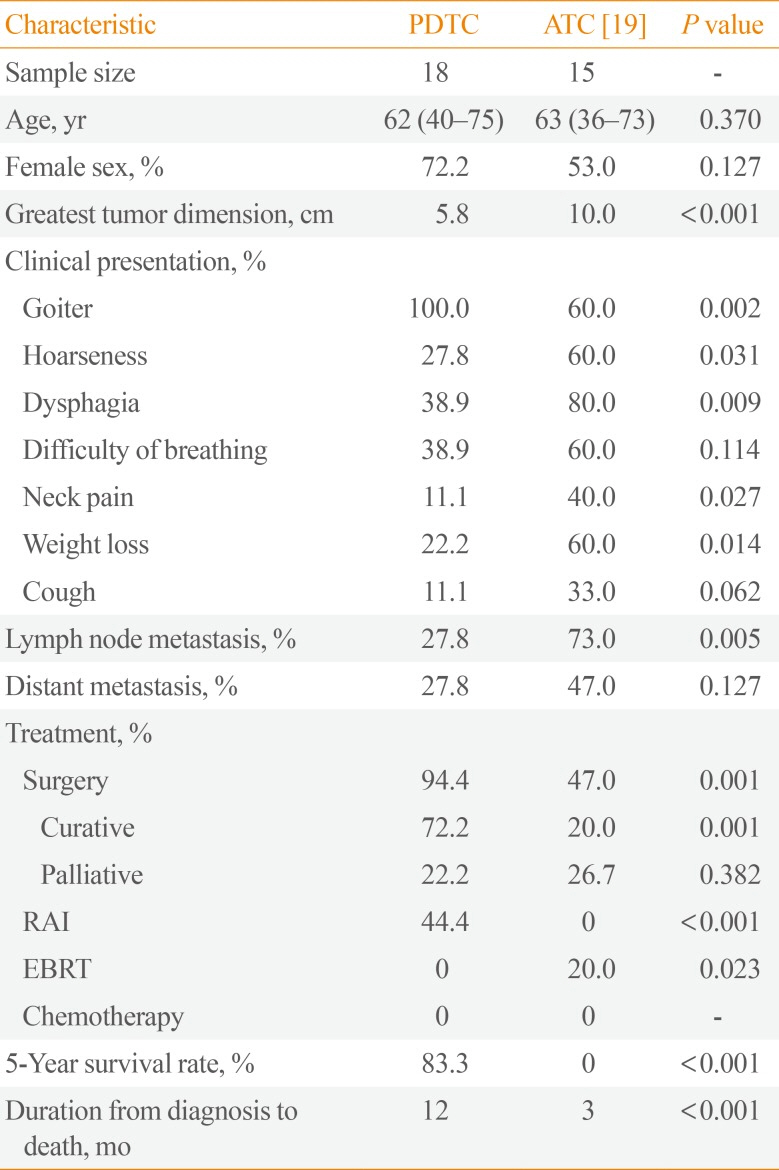
| Characteristic | PDTC | ATC [19] | P value |
|---|---|---|---|
| Sample size | 18 | 15 | - |
| Age, yr | 62 (40–75) | 63 (36–73) | 0.370 |
| Female sex, % | 72.2 | 53.0 | 0.127 |
| Greatest tumor dimension, cm | 5.8 | 10.0 | <0.001 |
| Clinical presentation, % | |||
| Goiter | 100.0 | 60.0 | 0.002 |
| Hoarseness | 27.8 | 60.0 | 0.031 |
| Dysphagia | 38.9 | 80.0 | 0.009 |
| Difficulty of breathing | 38.9 | 60.0 | 0.114 |
| Neck pain | 11.1 | 40.0 | 0.027 |
| Weight loss | 22.2 | 60.0 | 0.014 |
| Cough | 11.1 | 33.0 | 0.062 |
| Lymph node metastasis, % | 27.8 | 73.0 | 0.005 |
| Distant metastasis, % | 27.8 | 47.0 | 0.127 |
| Treatment, % | |||
| Surgery | 94.4 | 47.0 | 0.001 |
| Curative | 72.2 | 20.0 | 0.001 |
| Palliative | 22.2 | 26.7 | 0.382 |
| RAI | 44.4 | 0 | <0.001 |
| EBRT | 0 | 20.0 | 0.023 |
| Chemotherapy | 0 | 0 | - |
| 5-Year survival rate, % | 83.3 | 0 | <0.001 |
| Duration from diagnosis to death, mo | 12 | 3 | <0.001 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download