1. Heaney AP. Clinical review. Pituitary carcinoma: difficult diagnosis and treatment. J Clin Endocrinol Metab. 2011; 96:3649–3660. PMID:
21956419.
2. de Bruin C, Hofland LJ, Nieman LK, van Koetsveld PM, Waaijers AM, Sprij-Mooij DM, et al. Mifepristone effects on tumor somatostatin receptor expression in two patients with Cushing's syndrome due to ectopic adrenocorticotropin secretion. J Clin Endocrinol Metab. 2012; 97:455–462. PMID:
22090282.

3. Pivonello R, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, et al. Pasireotide treatment significantly improves clinical signs and symptoms in patients with Cushing's disease: results from a phase III study. Clin Endocrinol (Oxf). 2014; 81:408–417. PMID:
24533697.

4. Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, et al. A 12-month phase 3 study of pasireotide in Cushing's disease. N Engl J Med. 2012; 366:914–924. PMID:
22397653.

5. Gabalec F, Husek P, Pacovsky J, Cap J. Escape from response to long-term pasireotide treatment in recurrent Cushing's disease. Endocr Abstr. 2015; 37:EP716.

6. Lacroix A, Petersenn S, Biller BM, Arnaldez F, Roughton M, Ravichandran S, et al. SAT-546: Monthly pasireotide LAR improves urinary free cortisol (UFC) in patients with Cushing's disease: results from a randomized, double-blind, multicenter, phase III study. Poster presented at: Endocrine Society's 98th Annual Meeting and Expo. 2016 Apr 1-4; Boston, MA:
7. Burman P, Eden-Engstrom B, Ekman B, Karlsson FA, Schwarcz E, Wahlberg J. Limited value of cabergoline in Cushing's disease: a prospective study of a 6-week treatment in 20 patients. Eur J Endocrinol. 2016; 174:17–24. PMID:
26582653.

8. Godbout A, Manavela M, Danilowicz K, Beauregard H, Bruno OD, Lacroix A. Cabergoline monotherapy in the long-term treatment of Cushing's disease. Eur J Endocrinol. 2010; 163:709–716. PMID:
20702648.

9. Castinetti F, Guignat L, Giraud P, Muller M, Kamenicky P, Drui D, et al. Ketoconazole in Cushing's disease: is it worth a try? J Clin Endocrinol Metab. 2014; 99:1623–1630. PMID:
24471573.

10. Cuevas-Ramos D, Lim DS, Fleseriu M. Update on medical treatment for Cushing's disease. Clin Diabetes Endocrinol. 2016; 2:16.

11. Pivonello R, De Leo M, Cozzolino A, Colao A. The treatment of Cushing's disease. Endocr Rev. 2015; 36:385–486. PMID:
26067718.

12. Daniel E, Aylwin S, Mustafa O, Ball S, Munir A, Boelaert K, et al. Effectiveness of metyrapone in treating Cushing's syndrome: a retrospective multicenter study in 195 patients. J Clin Endocrinol Metab. 2015; 100:4146–4154. PMID:
26353009.

13. Baudry C, Coste J, Bou Khalil R, Silvera S, Guignat L, Guibourdenche J, et al. Efficiency and tolerance of mitotane in Cushing's disease in 76 patients from a single center. Eur J Endocrinol. 2012; 167:473–481. PMID:
22815335.

14. Molitch ME. Current approaches to the pharmacological management of Cushing's disease. Mol Cell Endocrinol. 2015; 408:185–189. PMID:
25450859.

15. Fleseriu M. Medical treatment of Cushing disease: new targets, new hope. Endocrinol Metab Clin North Am. 2015; 44:51–70. PMID:
25732642.
16. Fleseriu M, Pivonello R, Young J, Hamrahian AH, Molitch ME, Shimizu C, et al. Osilodrostat, a potent oral 11beta-hydroxylase inhibitor: 22-week, prospective, phase II study in Cushing's disease. Pituitary. 2016; 19:138–148. PMID:
26542280.
17. Fleseriu M, Castinetti F. Updates on the role of adrenal steroidogenesis inhibitors in Cushing's syndrome: a focus on novel therapies. Pituitary. 2016; 19:643–653. PMID:
27600150.

18. Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C, et al. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing's syndrome. J Clin Endocrinol Metab. 2012; 97:2039–2049. PMID:
22466348.

19. Fleseriu M, Findling JW, Koch CA, Schlaffer SM, Buchfelder M, Gross C. Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing's disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone. J Clin Endocrinol Metab. 2014; 99:3718–3727. PMID:
25013998.

20. Fleseriu M, Molitch ME, Gross C, Schteingart DE, Vaughan TB 3rd, Biller BM. A new therapeutic approach in the medical treatment of Cushing's syndrome: glucocorticoid receptor blockade with mifepristone. Endocr Pract. 2013; 19:313–326. PMID:
23337135.

21. Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of Cushing's syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015; 100:2807–2831. PMID:
26222757.

22. Cuevas-Ramos D, Fleseriu M. Treatment of Cushing’s disease: a mechanistic update. J Endocrinol. 2014; 223:R19–R39. PMID:
25134660.

23. Feelders RA, de Bruin C, Pereira AM, Romijn JA, Netea-Maier RT, Hermus AR, et al. Pasireotide alone or with cabergoline and ketoconazole in Cushing's disease. N Engl J Med. 2010; 362:1846–1848. PMID:
20463350.

24. Carmichael JD, Bonert VS, Nuno M, Ly D, Melmed S. Acromegaly clinical trial methodology impact on reported biochemical efficacy rates of somatostatin receptor ligand treatments: a meta-analysis. J Clin Endocrinol Metab. 2014; 99:1825–1833. PMID:
24606084.

25. Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014; 99:3933–3951. PMID:
25356808.

26. Caron PJ, Bevan JS, Petersenn S, Flanagan D, Tabarin A, Prevost G, et al. Tumor shrinkage with lanreotide Autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial. J Clin Endocrinol Metab. 2014; 99:1282–1290. PMID:
24423301.

27. Giustina A, Mazziotti G, Torri V, Spinello M, Floriani I, Melmed S. Meta-analysis on the effects of octreotide on tumor mass in acromegaly. PLoS One. 2012; 7:e36411. PMID:
22574156.

28. Kiseljak-Vassiliades K, Carlson NE, Borges MT, Kleinschmidt-DeMasters BK, Lillehei KO, Kerr JM, et al. Growth hormone tumor histological subtypes predict response to surgical and medical therapy. Endocrine. 2015; 49:231–241. PMID:
25129651.

29. Cuevas-Ramos D, Fleseriu M. Somatostatin receptor ligands and resistance to treatment in pituitary adenomas. J Mol Endocrinol. 2014; 52:R223–R240. PMID:
24647046.

30. Potorac I, Petrossians P, Daly AF, Alexopoulou O, Borot S, Sahnoun-Fathallah M, et al. T2-weighted MRI signal predicts hormone and tumor responses to somatostatin analogs in acromegaly. Endocr Relat Cancer. 2016; 23:871–881. PMID:
27649724.

31. Colao A, Auriemma RS, Lombardi G, Pivonello R. Resistance to somatostatin analogs in acromegaly. Endocr Rev. 2011; 32:247–271. PMID:
21123741.

32. Fleseriu M. Advances in the pharmacotherapy of patients with acromegaly. Discov Med. 2014; 17:329–338. PMID:
24979253.
33. Vilar L, Fleseriu M, Naves LA, Albuquerque JL, Gadelha PS, dos Santos Faria M, et al. Can we predict long-term remission after somatostatin analog withdrawal in patients with acromegaly? Results from a multicenter prospective trial. Endocrine. 2014; 46:577–584. PMID:
24272601.

34. Casagrande A, Bronstein MD, Jallad RS, Moraes AB, Elias PC, Castro M, et al. Long-term remission of acromegaly after octreotide withdrawal is an uncommon and frequently unsustainable event. Neuroendocrinology. 2017; 104:273–279. PMID:
27161443.

35. Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014; 2:875–884. PMID:
25260838.

36. Iacovazzo D, Carlsen E, Lugli F, Chiloiro S, Piacentini S, Bianchi A, et al. Factors predicting pasireotide responsiveness in somatotroph pituitary adenomas resistant to first-generation somatostatin analogues: an immunohistochemical study. Eur J Endocrinol. 2016; 174:241–250. PMID:
26586796.

37. Schmid HA, Brue T, Colao A, Gadelha MR, Shimon I, Kapur K, et al. Effect of pasireotide on glucose- and growth hormone-related biomarkers in patients with inadequately controlled acromegaly. Endocrine. 2016; 53:210–219. PMID:
26906713.

38. Sandret L, Maison P, Chanson P. Place of cabergoline in acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2011; 96:1327–1335. PMID:
21325455.

39. Kuhn E, Chanson P. Cabergoline in acromegaly. Pituitary. 2017; 20:121–128. PMID:
28025719.

40. van der Lely AJ, Biller BM, Brue T, Buchfelder M, Ghigo E, Gomez R, et al. Long-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDY. J Clin Endocrinol Metab. 2012; 97:1589–1597. PMID:
22362824.

41. Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000; 342:1171–1177. PMID:
10770982.

42. Droste M, Domberg J, Buchfelder M, Mann K, Schwanke A, Stalla G, et al. Therapy of acromegalic patients exacerbated by concomitant type 2 diabetes requires higher pegvisomant doses to normalise IGF1 levels. Eur J Endocrinol. 2014; 171:59–68. PMID:
24913198.

43. Lim DS, Fleseriu M. The role of combination medical therapy in the treatment of acromegaly. Pituitary. 2017; 20:136–148. PMID:
27522663.

44. Franck SE, Muhammad A, van der Lely AJ, Neggers SJ. Combined treatment of somatostatin analogues with pegvisomant in acromegaly. Endocrine. 2016; 52:206–213. PMID:
26661938.

45. Melmed S, Popovic V, Bidlingmaier M, Mercado M, van der Lely AJ, Biermasz N, et al. Safety and efficacy of oral octreotide in acromegaly: results of a multicenter phase III trial. J Clin Endocrinol Metab. 2015; 100:1699–1708. PMID:
25664604.

46. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:273–288. PMID:
21296991.

47. Noronha S, Stokes V, Karavitaki N, Grossman A. Treating prolactinomas with dopamine agonists: always worth the gamble? Endocrine. 2016; 51:205–210. PMID:
26336835.

48. Molitch ME. Management of medically refractory prolactinoma. J Neurooncol. 2014; 117:421–428. PMID:
24146188.

49. Ono M, Miki N, Kawamata T, Makino R, Amano K, Seki T, et al. Prospective study of high-dose cabergoline treatment of prolactinomas in 150 patients. J Clin Endocrinol Metab. 2008; 93:4721–4727. PMID:
18812485.

50. Drake WM, Stiles CE, Bevan JS, Karavitaki N, Trainer PJ, Rees DA, et al. A follow-up study of the prevalence of valvular heart abnormalities in hyperprolactinemic patients treated with cabergoline. J Clin Endocrinol Metab. 2016; 101:4189–4194. PMID:
27571182.

51. Liu X, Kano M, Araki T, Cooper O, Fukuoka H, Tone Y, et al. ErbB receptor-driven prolactinomas respond to targeted lapatinib treatment in female transgenic mice. Endocrinology. 2015; 156:71–79. PMID:
25375038.

52. Cooper O, Mamelak A, Bannykh S, Carmichael J, Bonert V, Lim S, et al. Prolactinoma ErbB receptor expression and targeted therapy for aggressive tumors. Endocrine. 2014; 46:318–327. PMID:
24287797.

53. Gagliano T, Filieri C, Minoia M, Buratto M, Tagliati F, Ambrosio MR, et al. Cabergoline reduces cell viability in non functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Pituitary. 2013; 16:91–100. PMID:
22350942.

54. Colao A, Di Somma C, Pivonello R, Faggiano A, Lombardi G, Savastano S. Medical therapy for clinically non-functioning pituitary adenomas. Endocr Relat Cancer. 2008; 15:905–915. PMID:
18780796.

55. Garcia EC, Naves LA, Silva AO, de Castro LF, Casulari LA, Azevedo MF. Short-term treatment with cabergoline can lead to tumor shrinkage in patients with nonfunctioning pituitary adenomas. Pituitary. 2013; 16:189–194. PMID:
22740242.

56. Greenman Y, Cooper O, Yaish I, Robenshtok E, Sagiv N, Jonas-Kimchi T, et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur J Endocrinol. 2016; 175:63–72. PMID:
27150495.

57. Sav A, Rotondo F, Syro LV, Di Ieva A, Cusimano MD, Kovacs K. Invasive, atypical and aggressive pituitary adenomas and carcinomas. Endocrinol Metab Clin North Am. 2015; 44:99–104. PMID:
25732646.

58. Ji Y, Vogel RI, Lou E. Temozolomide treatment of pituitary carcinomas and atypical adenomas: systematic review of case reports. Neurooncol Pract. 2016; 3:188–195. PMID:
27551432.

59. Whitelaw BC, Dworakowska D, Thomas NW, Barazi S, Riordan-Eva P, King AP, et al. Temozolomide in the management of dopamine agonist-resistant prolactinomas. Clin Endocrinol (Oxf). 2012; 76:877–886. PMID:
22372583.

60. Ceccato F, Lombardi G, Manara R, Emanuelli E, Denaro L, Milanese L, et al. Temozolomide and pasireotide treatment for aggressive pituitary adenoma: expertise at a tertiary care center. J Neurooncol. 2015; 122:189–196. PMID:
25555563.

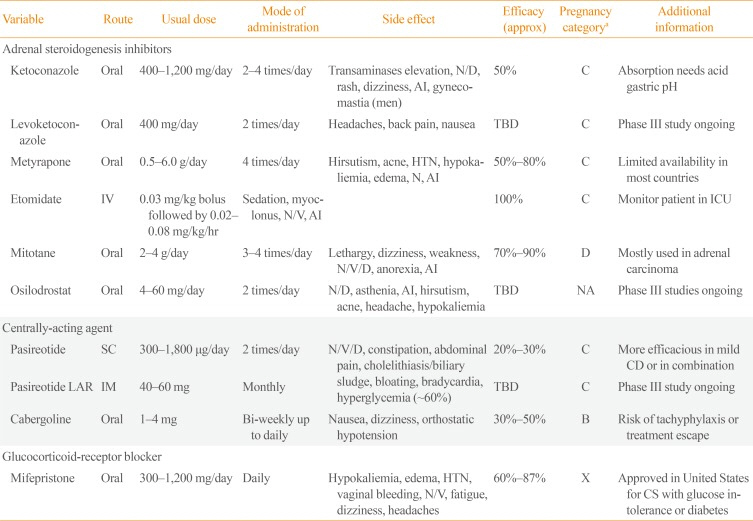
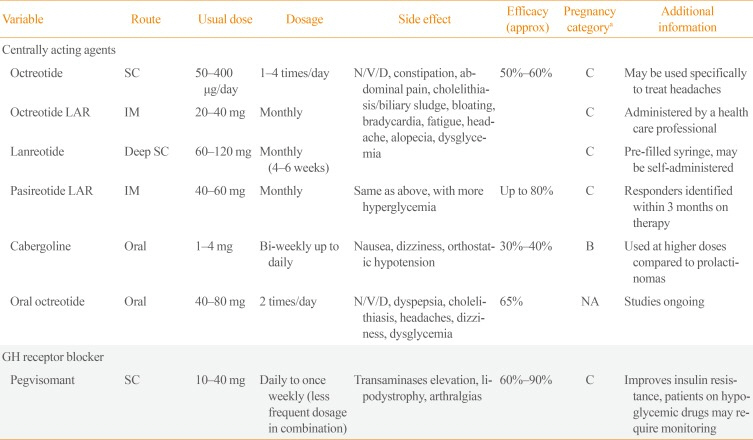
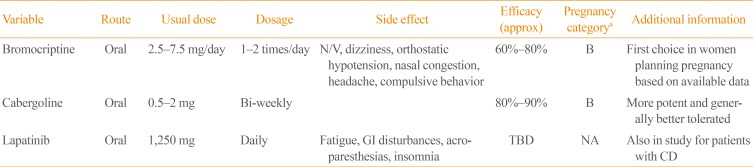




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download