Abstract
Background
Renal tubulointerstitial fibrosis is a common feature of the final stage of nearly all cause types of chronic kidney disease. Although classic peroxisome proliferator-activated receptor γ (PPARγ) agonists have a protective effect on diabetic nephropathy, much less is known about their direct effects in renal fibrosis. This study aimed to investigate possible beneficial effects of lobeglitazone, a novel PPARγ agonist, on renal fibrosis in mice.
Methods
We examined the effects of lobeglitazone on renal tubulointerstitial fibrosis in unilateral ureteral obstruction (UUO) induced renal fibrosis mice. We further defined the role of lobeglitazone on transforming growth factor (TGF)-signaling pathways in renal tubulointerstitial fibrosis through in vivo and in vitro study.
Results
Through hematoxylin/eosin and sirius red staining, we observed that lobeglitazone effectively attenuates UUO-induced renal atrophy and fibrosis. Immunohistochemical analysis in conjunction with quantitative reverse transcription polymerase chain reaction and Western blot analysis revealed that lobeglitazone treatment inhibited UUO-induced upregulation of renal Smad-3 phosphorylation, α-smooth muscle actin, plasminogen activator inhibitor 1, and type 1 collagen. In vitro experiments with rat mesangial cells and NRK-49F renal fibroblast cells suggested that the effects of lobeglitazone on UUO-induced renal fibrosis are mediated by inhibition of the TGF-β/Smad signaling pathway.
The incidence of chronic kidney disease (CKD) has increased in recent years, with more people suffering from end stage renal failure [1]. A common pathologic feature of end stage kidney disease is renal tubulointerstitial fibrosis characterized by transforming growth factor β (TGF-β)/Smad signaling-mediated extracellular matrix (ECM) accumulation [23]. TGF-β/Smad signaling is a master regulatory pathway of profibrotic genes such as α-smooth muscle actin (α-SMA), plasminogen activator inhibitor 1 (PAI-1), and type 1 collagen [45]. For this reason, much effort has been placed into finding an effective strategy for inhibiting TGF-β/Smad signaling to treat renal tubulointerstitial fibrosis [67].
Thiazolidinediones (TZDs), synthetic peroxisome proliferator-activated receptor γ (PPARγ) agonists, are often used to manage type 2 diabetes mellitus (T2DM) via regulation of glucose and lipid metabolism [8]. TZDs also affect a diverse range of activities including cell proliferation, apoptosis, inflammation, and oxidative stress responses [910]. Recent studies have demonstrated beneficial effects of TZD on various renal injuries. Treatment with PPARγ agonists has protective effects against both diabetic and non-diabetic origin CKD [1112]. Although glycemic and lipid control can contribute to their protective renal effect, recent evidence suggests that upregulation of PPARγ expression in the kidney itself provides additional renal benefits by reducing TGF-β-induced ECM production, maintaining podocyte numbers and function, and regulating inflammatory cell infiltration [1314].
Lobeglitazone is a new PPARγ agonist with a TZD moiety and substituted pyrimidines, currently used to treat T2DM after completing clinical trials [15]. Phase III clinical trial data show that lobeglitazone treatment resulted in an approximately 0.6% to 0.74% decrease in glycated hemoglobin compared with that of the placebo [1617]. It is administered as a once-daily dose and mainly excreted in feces, reducing concerns of bladder cancer unlike the classic TZD pioglitazone. As another antidiabetic agent, it is necessary to evaluate additional effects of lobeglitazone on diabetic micro/macrovascular complications. A recent study has provided evidence on the cardiovascular protective role of lobeglitazone in the proliferation and migration of vascular cells. In the balloon injury rat model, lobeglitazone-treated rats showed less neointimal formation in the carotid artery than placebo-treated rats. Lobeglitazone treatment also reduced the atheromatous burden in the aorta of apolipoprotein E knockout mice fed a high-fat and high cholesterol diet [18]. However, there are no human or animal studies on any potential renal protective effects of lobeglitazone. The effects of lobeglitazone on renal tubulointerstitial fibrosis have also not been studied.
In the present study, we evaluated whether lobeglitazone had antifibrotic effects on renal tubulointerstitial disease in unilateral ureteral obstruction (UUO) mice, a model of renal tubulointerstitial fibrosis. We also examined the antifibrotic properties of lobeglitazone in vitro.
C57BL6 mice were pretreated with 1 mg/kg lobeglitazone (Chung Kun Dang Pharmaceutical Corp., Seoul, Korea) by gavage daily for 3 days. For UUO-induced renal fibrosis, the left ureter of mice was doubly ligated. UUO was performed as previously described [19]. After UUO, C57BL6 mice were treated with 1 mg/kg lobeglitazone by gavage for 7 days consecutively. Seven days after UUO and lobeglitazone treatment, mice were euthanized, and their left kidneys were removed, cut in thirds, fixed in 4% paraformaldehyde, and either embedded in paraffin for histologic examination or frozen in liquid nitrogen for the isolation of protein or RNA. All procedures were performed in accordance with institutional guidelines for animal research [6].
Histologic and morphologic analysis was performed as previously described [19]. Histochemical staining was performed with hematoxylin/eosin and sirius red. Immunohistochemical staining was performed using primary antibodies against p-Smad3 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), α-SMA (1:500; Sigma, St. Louis, MO, USA), PAI-1 (1:500; BD Biosciences, San Jose, CA, USA), and type 1 collagen (1:500; Abcam, Cambridge, UK), followed by horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G secondary antibodies (Dako, Glostrup, Denmark). Quantification of renal fibrosis was measured as previously described [19].
NRK-49F normal rat kidney fibroblasts and rat mesangial cells (RMCs) were purchased from the American Type Culture Collection (Manassas, VA, USA). NRK-49F cells were cultured in 5% CO2/95% air at 37℃ in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Grand Island, NY, USA) supplemented with 5% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) and antibiotics. RMCs were cultured in 5% CO2/95% air at 37℃ in DMEM. The medium was supplemented with 15% FBS and 0.4 mg/mL G418. Cells were rendered quiescent by incubation for 24 hours in medium supplemented with 0.5% FBS. Cells were treated with medium containing 0.5% FBS with or without TGF-β (5 ng/mL; R&D Systems, Minneapolis, MN, USA) for 24 hours. Cells were incubated with lobeglitazone (10 µM) for 24 hours. Cells were subsequently processed for the isolation of RNA or protein as described below.
Western blot was performed as previously described [19]. Membranes were incubated with anti-p-Smad3 (1:1,000; Cell Signaling Technology, Danvers, MA, USA), anti-Smad3 (1:1,000; Cell Signaling Technology), anti-PAI-1 (1:1,000; BD Biosciences), anti-α-SMA (1:1,000; Sigma), and anti-type I collagen (1:1,000; Abcam) polyclonal antibodies at 4℃ with gentle shaking overnight. Antibodies were detected by horseradish peroxidase-linked secondary antibody (Santa Cruz) using an Enhanced Chemiluminescence Western Blotting Detection System, in accordance with the manufacturer's instructions (Millipore, Billerica, MA, USA) [19]. The membrane was reblotted with anti-β-tubulin antibody (Applied Biological Materials Inc., Richmond, BC, Canada) to verify equal protein loading in each lane. Densitometric measurements of the bands were performed using UN-SCAN-IT digitizing software (Silk Scientific Corp., Orem, UT, USA).
Total RNA isolation and quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) was performed as previously described [19]. Primers were designed using AB StepOne software version 2.1 (Applied Biosystems, Foster City, CA, USA) and were based on the relevant sequences from GenBank as follows: mouse α-SMA (GenBank accession NM_007392.3; sense, 5´-CAGGCTGTGCTGTCCCTCTA-3´; antisense, 5´-CGGCAGTAGTCACGAAGGAA-3´), mouse PAI-1 (GenBank accession NM_008871.2; sense, 5´-AATCCCACACAGCCCATCA-3´; antisense, 5´-GGACCACCTGCTGAAACACTTT-3´), mouse type 1 collagen (GenBank accession NM_ 007742.3; sense, 5´-GCCTTGGAGGAAACTTTGCTT-3´; antisense, 5´-GCACGGAAACTCCAGCTGAT-3´), mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (GenBank accession NM_008084.2; sense, 5´-GAAGGGTGGAGCCAAAA G-3´; antisense, 5´-GCTGACAATCTTGAGTGAGT-3´), rat α-SMA (GenBank accession NM_031004.2; sense, 5´-GCACTACCATGTACCCAGGCAT-3´; antisense, 5´-TGCGTTCTGGAGGAGCAATAA-3´), ratPAI-1 (GenBank accession NM_012620.1; sense, 5´-CACCCCTTCCAGAGTCCCATA-3´; antisense, 5´-GCTGAAACACTTTTACTCCGAAGTT-3´), rat type 1 collagen (GenBank accession NM_053304.1; sense, 5´-GTGCGATGGCGTGCTATG-3´; antisense, 5´-TCGCCCTCCCGTTTTTG-3´), and rat GAPDH (GenBank accession NM_017008.4; sense, 5´-TGCCGCCTGGAGAAACC-3´; antisense, 5´-AGCCCAAGGATGCCCTTTAGT-3´). The housekeeping gene GAPDH was used as an internal control.
Transient transfection and reporter assay was performed as previously described [20].
The effects of lobeglitazone on renal tubulointerstitial fibrosis were evaluated using the UUO model. As shown in Fig. 1A, hematoxylin/ eosin and sirius red staining showed that vehicle-treated UUO kidneys exhibited prominent renal tubular atrophy and tubulointerstitial fibrosis. By contrast, lobeglitazone-treated UUO kidneys showed marked attenuation of UUO-induced tubular atrophy and tubulointerstitial fibrosis (Fig. 1A).
Given that TGF-β/Smad3 is a well-known mediator in the development of renal tubulointerstitial fibrosis, we examined the effects of lobeglitazone on the levels of Smad3 phosphorylation and Smad3 target genes including α-SMA, PAI-1, and type 1 collagen. The results showed that positively stained areas for phosphorylated Smad3, α-SMA, PAI-1, and type 1 collagen were evidently increased in the damaged tubules of UUO kidneys, but these were significantly reduced by lobeglitazone treatment (Fig. 1B).
The effects of lobeglitazone on fibrotic gene expression were further confirmed by quantitative RT-PCR and Western blot analysis. Consistent with the immunohistochemical analysis, the protein abundance of PAI-1, α-SMA, and type 1 collagen was lower in the kidneys of mice administered lobeglitazone than in vehicle-treated kidneys (Fig. 2A). Moreover, UUO-induced Smad3 phosphorylation was markedly suppressed in the kidneys of lobeglitazone-treated mice (Fig. 2A). The mRNA expression levels of these genes in the kidneys of lobeglitazone-treated mice were also markedly lower than in vehicle-treated mice (Fig. 2B).
To examine the mechanism responsible for the antifibrotic effects of lobeglitazone, we examined whether lobeglitazone inhibits TGF-β-stimulated Smad3 signaling in cultured renal cells including NRK-49F cells and RMCs. As expected, TGF-β treatment increased mRNA and protein levels of PAI-1, α-SMA, and type 1 collagen, and induced Smad3 phosphorylation. Lobeglitazone-treated NRK-49F cells showed markedly inhibited TGF-β-stimulated profibrotic gene expression and Smad3 phosphorylation (Fig. 3A, B). Consistent with the effects in NRK-49F cells, lobeglitazone also suppressed TGF-β-stimulated Smad3 phosphorylation, PAI-1, α-SMA, and type 1 collagen in RMCs (Fig. 3C, D).
To determine whether lobeglitazone-induced suppression of profibrotic gene expression is mediated at the transcriptional level, we examined whether lobeglitazone treatment inhibits TGF-β/Smad-stimulated PAI-1 promoter activity. Indeed, lobeglitazone treatment successfully inhibited TGF-β and ALK5/Smad3, 4-stimulated PAI-1 promoter activity both in NRK-49F cells and RMCs (Fig. 4). These results indicate that lobeglitazone has an antifibrotic effect through the inhibition of TGF-β-stimulated Smad3 transcriptional activity on its target genes.
The study presented here shows that lobeglitazone treatment attenuates renal fibrosis in UUO mice. Lobeglitazone inhibited UUO-induced profibrotic gene expression including PAI-1, α-SMA, and type 1 collagen. The antifibrotic effects of lobeglitazone were associated with inhibition of the TGF-β/Smad3 signaling pathway.
PPARγ agonists are widely used as antidiabetic agents through improved insulin sensitivity and lipid metabolism [21]. On the basis of their wide range of metabolic benefits, PPARγ agonists can ameliorate diabetic nephropathy in animal and human studies [22]. Recently, several lines of evidence show that PPARγ agonists can reduce acute kidney injury, indicating that their renal protective properties may be partially independent of metabolic factors. For instance, activation of PPARγ reduces glomerulosclerosis and apoptosis via regulation of inflammation in various animal models of non-diabetic nephropathy including 5/6 nephrectomy [12], passive Heymann nephritis [23], cisplatin-induced renal damage [24], and ischemia/reperfusion injury [25]. Additionally, rosiglitazone, pioglitazone, and troglitazone have a role in reducing renal tubulointerstitial fibrosis in the UUO model [26272829]. Rosiglitazone inhibits renal tubulointerstitial fibrosis through inhibiting interstitial macrophage infiltration, downregulating the expressions of TGF-β and its down-stream target genes, and up-regulating the BMP-7 expression [26]. Several groups examined antifibrotic effects of pioglitazone and interaction with angiotensin receptor antagonists such as L158809 and candesartan in the UUO model [2728]. Pioglitazone and candesartan have additive protective effects on renal fibrosis, but the synergism between pioglitazone and L158809 is not clear [27]. Troglitazone attenuates renal interstitial fibrosis and inflammation in dose dependent manner by down regulation of TGF-β signaling pathway in the model of UUO. In accordance with these findings, our present study showed that lobeglitazone also has a protective effect on renal tubulointerstitial fibrosis and UUO-induced tubular atrophy. Furthermore, lobeglitazone inhibited the expression of well-known TGF-β target genes, such as PAI-1, α-SMA, and type 1 collagen, as well as its major effector, phosphorylated Smad3, in the kidneys of UUO mice.
The TGF-β/Smad signaling pathway is a primary pathogenic factor that drives glomerular and tubulointerstitial fibrosis in the kidney by stimulating the synthesis of ECM molecules and by decreasing ECM degradation [4]. Although mesangial cells express Smad1, 2, 3, 4, and 7, accumulating evidence demonstrates that Smad3 is mainly implicated in a pathogenic role in TGF-β-mediated renal fibrosis [303132]. Recent studies show that several PPARγ agonists inhibit TGF-β-stimulated ECM production, indicating that PPARγ agonists attenuate renal tubulointerstitial fibrosis by inhibiting the TGF-β/Smad3 signaling pathway [33]. Pioglitazone inhibits renal tubulointerstitial fibrosis and infiltration of interstitial macrophages by regulating transcription of PAI-1in UUO mice [28]. Rosiglitazone treatment inhibits inflammatory reactions and renal fibrosis by reducing the overexpression of endogenous endothelin-1, cyclooxygenase-2, and TGF-β in deoxycorticosterone acetate-salt hypertensive rats [34]. Our study adds evidence that another novel PPARγ agonist, lobeglitazone suppresses tubulointerstitial fibrosis after UUO in mice by inhibiting TGF-β/Smad3 signaling.
In conclusion, this study demonstrates that lobeglitazone has a renoprotective effect on UUO-induced renal fibrosis through inhibition of the TGF-β/Smad3 pathway. Our results suggest that lobeglitazone could play a therapeutic role in CKD, providing rationale for further clinical trials to evaluate the efficacy of lobeglitazone in the treatment of CKD including renal tubulointerstitial fibrosis.
Figures and Tables
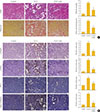 | Fig. 1Effects of lobeglitazone on unilateral ureteral obstruction (UUO)-induced renopathological changes. (A) Representative images of hematoxylin and eosin (H&E) and sirius red staining of kidney tissue sections from control (CON) mice and UUO mice with or without lobeglitazone (Lobe; 1 mg/kg) treatment. The number of atrophic tubules was determined by measuring abnormal and dilated tubular basement membranes in five random fields of H&E stained sections under high power magnification (×200). Areas of positive staining with sirius red were quantitated by computer-based morphometric analysis. All morphometric data were normalized against the corresponding values in CON animals. Data in all bar graphs are expressed as fold increase relative to the CON (n=6 in each group). (B) Representative images of immunohistochemical staining forp-Smad3, α-smooth muscle actin (α-SMA), plasminogen activator inhibitor 1 (PAI-1), and type I collagen in kidney tissue sections from CON mice or UUO mice with or without lobeglitazone (1 mg/kg). Areas of positive staining with p-Smad3, α-SMA, PAI-1, and type 1 collagen antibodies were quantitated by computer-based morphometric analysis. All data were expressed as the mean±SEM of five random fields from each kidney section (n=6 in each group). aP<0.05; bP<0.01; cP<0.001 vs. CON; and dP<0.05; eP<0.01; fP<0.001 vs. UUO. |
 | Fig. 2Effects of lobeglitazone on profibrotic gene expression in kidneys of unilateral ureteral obstruction (UUO) mice. (A) Representative Western blot analysis of p-Smad3, t-Smad3, α-smooth muscle actin (α-SMA), plasminogen activator inhibitor 1 (PAI-1), and type 1 collagen protein expression in UUO kidneys with or without lobeglitazone (Lobe; 1 mg/kg; n=6 in each group). Data are expressed as the mean±SEM of three independent experiments. (B) Representative real-time reverse transcription polymerase chain reaction analysis of α-SMA, PAI-1, and type 1 collagen mRNA expression in UUO kidneys with or without Lobe (1 mg/kg; n=6 in each group). Data in bar graphs are mean±SEM. aP<0.05; bP<0.01; cP<0.001 vs. control (CON); and dP<0.05; eP<0.01; fP<0.001 vs. UUO. |
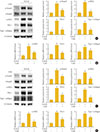 | Fig. 3Effects of lobeglitazone (Lobe) on transforming growth factor β (TGF-β)-induced profibrotic gene expression in cultured kidney cell lines. Representative Western blot analysis (A) of p-Smad3, t-Smad3, α-smooth muscle actin (α-SMA), plasminogen activator inhibitor 1 (PAI-1), and type 1 collagen levels and representative real-time reverse transcription polymerase chain reaction (RT-PCR) analysis (B) of α-SMA, PAI-1, and type 1 collagen expression in TGF-β-stimulated NRK-49F cells. Representative Western blot analysis (C) of p-Smad3, t-Smad3, α-SMA, PAI-1, and type 1 collagen expression and representative real-time RT-PCR analysis (D) of α-SMA, PAI-1, and type 1 collagen expression in TGF-β-stimulated rat mesangial cells. Cells were co-incubated with TGF-β (5 ng/mL) and Lobe (10 µM) after 24 hours serum starvation. Data are the mean±SEM of three independent measurements (three separate experiments). aP<0.05; bP<0.01; cP<0.001 vs. control (CON); and dP<0.05; eP<0.01; fP<0.001 vs. TGF-β alone. |
 | Fig. 4Effects of lobeglitazone (Lobe) on the transforming growth factor β (TGF-β)/Smad3 signaling pathway in kidney cell lines. (A) Effects of Lobe on plasminogen activator inhibitor 1 (PAI-1) promoter activity in NRK-49F cells. Cells were treated with TGF-β (5 ng/mL) with or without Lobe co-treatment (10 µM) for 24 hours (left panel). (B) Effects of Lobe on PAI-1 promoter activity in rat mesangial cells. Cells were treated with TGF-β (5 ng/mL) with or without Lobe co-treatment (10 µM) for 24 hours (left panel). Cells were co-transfected with the PAI-1 promoter and expression vectors for Smad3/4 (pRK5) and ALK5 (pcDNA) with or without Lobe treatment (10 µM) for 24 hours (right panel). Data are the mean±SEM of three independent measurements. aP<0.01; bP<0.001 vs. control; and cP<0.001 vs. TGF-β alone or vs. Smad3/4 and ALK5. |
ACKNOWLEDGMENTS
This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2016).
References
1. Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005; 365:331–340.
2. Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010; 21:1819–1834.
3. Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006; 17:17–25.
4. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016; 12:325–338.
5. Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006; 17:2999–3012.
6. Jung GS, Kim MK, Jung YA, Kim HS, Park IS, Min BH, et al. Clusterin attenuates the development of renal fibrosis. J Am Soc Nephrol. 2012; 23:73–85.
7. Won JC, Park CY, Oh SW, Lee ES, Youn BS, Kim MS. Plasma clusterin (ApoJ) levels are associated with adiposity and systemic inflammation. PLoS One. 2014; 9:e103351.
8. Derosa G, Maffioli P. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) agonists on glycemic control, lipid profile and cardiovascular risk. Curr Mol Pharmacol. 2012; 5:272–281.
9. Cunard R, Ricote M, DiCampli D, Archer DC, Kahn DA, Glass CK, et al. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. J Immunol. 2002; 168:2795–2802.
10. Guan Y, Zhang Y, Breyer MD. The role of PPARs in the transcriptional control of cellular processes. Drug News Perspect. 2002; 15:147–154.
11. Smith SA, Lister CA, Toseland CD, Buckingham RE. Rosiglitazone prevents the onset of hyperglycaemia and proteinuria in the Zucker diabetic fatty rat. Diabetes Obes Metab. 2000; 2:363–372.
12. Ma LJ, Marcantoni C, Linton MF, Fazio S, Fogo AB. Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int. 2001; 59:1899–1910.
13. Routh RE, Johnson JH, McCarthy KJ. Troglitazone suppresses the secretion of type I collagen by mesangial cells in vitro. Kidney Int. 2002; 61:1365–1376.
14. Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M. Peroxisome proliferator-activated receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin expression in glomerular mesangial cells. Diabetes. 2004; 53:200–208.
15. Kim JW, Kim JR, Yi S, Shin KH, Shin HS, Yoon SH, et al. Tolerability and pharmacokinetics of lobeglitazone (CKD-501), a peroxisome proliferator-activated receptor-gamma agonist: a single- and multiple-dose, double-blind, randomized control study in healthy male Korean subjects. Clin Ther. 2011; 33:1819–1830.
16. Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, Ko KS, et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS One. 2014; 9:e92843.
17. Jin SM, Park CY, Cho YM, Ku BJ, Ahn CW, Cha BS, et al. Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension. Diabetes Obes Metab. 2015; 17:599–602.
18. Lim S, Lee KS, Lee JE, Park HS, Kim KM, Moon JH, et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015; 243:107–119.
19. Jung GS, Jeon JH, Choe MS, Kim SW, Lee IK, Kim MK, et al. Renoprotective effect of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in streptozotocin-induced type 1 diabetic mice. Diabetes Metab J. 2016; 40:211–221.
20. Jung GS, Kim MK, Choe MS, Lee KM, Kim HS, Park YJ, et al. The orphan nuclear receptor SHP attenuates renal fibrosis. J Am Soc Nephrol. 2009; 20:2162–2170.
21. Kim H, Haluzik M, Gavrilova O, Yakar S, Portas J, Sun H, et al. Thiazolidinediones improve insulin sensitivity in adipose tissue and reduce the hyperlipidaemia without affecting the hyperglycaemia in a transgenic model of type 2 diabetes. Diabetologia. 2004; 47:2215–2225.
22. Pistrosch F, Passauer J, Herbrig K, Schwanebeck U, Gross P, Bornstein SR. Effect of thiazolidinedione treatment on proteinuria and renal hemodynamic in type 2 diabetic patients with overt nephropathy. Horm Metab Res. 2012; 44:914–918.
23. Benigni A, Zoja C, Tomasoni S, Campana M, Corna D, Zanchi C, et al. Transcriptional regulation of nephrin gene by peroxisome proliferator-activated receptor-gamma agonist: molecular mechanism of the antiproteinuric effect of pioglitazone. J Am Soc Nephrol. 2006; 17:1624–1632.
24. Jesse CR, Bortolatto CF, Wilhelm EA, Roman SS, Prigol M, Nogueira CW. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone protects against cisplatin-induced renal damage in mice. J Appl Toxicol. 2014; 34:25–32.
25. Doi S, Masaki T, Arakawa T, Takahashi S, Kawai T, Nakashima A, et al. Protective effects of peroxisome proliferator-activated receptor gamma ligand on apoptosis and hepatocyte growth factor induction in renal ischemia-reperfusion injury. Transplantation. 2007; 84:207–213.
26. Lin Q, Gu Y, Ma J, Lin SY. Protection of rosiglitazone against renal interstitial lesion and its mechanism. Zhonghua Yi Xue Za Zhi. 2005; 85:1618–1624.
27. Han JY, Kim YJ, Kim L, Choi SJ, Park IS, Kim JM, et al. PPARgamma agonist and angiotensin II receptor antagonist ameliorate renal tubulointerstitial fibrosis. J Korean Med Sci. 2010; 25:35–41.
28. Higashi K, Oda T, Kushiyama T, Hyodo T, Yamada M, Suzuki S, et al. Additive antifibrotic effects of pioglitazone and candesartan on experimental renal fibrosis in mice. Nephrology (Carlton). 2010; 15:327–335.
29. Kawai T, Masaki T, Doi S, Arakawa T, Yokoyama Y, Doi T, et al. PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Lab Invest. 2009; 89:47–58.
30. Lan HY, Chung AC. TGF-beta/Smad signaling in kidney disease. Semin Nephrol. 2012; 32:236–243.
31. Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, et al. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol. 2010; 21:1477–1487.
32. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004; 18:816–827.
33. Ohtomo S, Izuhara Y, Takizawa S, Yamada N, Kakuta T, van Ypersele de Strihou C, et al. Thiazolidinediones provide better renoprotection than insulin in an obese, hypertensive type II diabetic rat model. Kidney Int. 2007; 72:1512–1519.
34. Bae EH, Kim IJ, Ma SK, Kim SW. Rosiglitazone prevents the progression of renal injury in DOCA-salt hypertensive rats. Hypertens Res. 2010; 33:255–262.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download