Abstract
Background
Tumor associated macrophages (TAMs) and CXC chemokine receptor 4 (CXCR4) have emerged as potential biomarkers in various human cancers. The aims of this study were to investigate the clinical characteristics of anaplastic thyroid cancer (ATC) patients according to the TAM numbers in the tumor tissue, and to evaluate the associations between CXCR4 expressions and macrophage densities in ATC tumor microenvironment.
Methods
Total 14 ATC samples from thyroid tissue microarray were used. Immunohistochemical staining was performed using anti-CD163 and anti-CXCR4 antibodies. According to the immunoreactivity of CD163, all subjects were divided into two groups: low-CD163 (n=8) and high-CD163 (n=6) groups.
Results
The mean diagnostic age was 65±7 years and the median tumor size was 4.3 cm, ranging 2.5 to 15 cm. Clinicopathological characteristics were not significantly different between low-CD163 and high-CD163 groups, while age of diagnosis was younger in high-CD163 group than that of low-CD163 group with marginal significance (56.9±5.5 years vs. 67.5±6.8 years, P=0.09). However, overall survival was significantly reduced in high-CD163 group (5.5 months [range, 1 to 10]) compared with low-CD163 groups (8.8 months [range, 6 to 121); log-rank test, P=0.0443). Moreover, high-CD163 group showed strong CXCR4 expressions in both cancer and stromal compartments, while low-CD163 group showed relatively weak, stromal-dominant CXCR4 expressions. Additionally, CD163 and CXCR4 expressions showed a strong positive correlation (γ2=0.432, P=0.013).
Anaplastic thyroid cancer (ATC) is the most virulent and aggressive cancer in human solid cancers, showing extremely short survival outcomes [1]. The median survival time ranges from 2.5 to 6 months, and the 5-year survival rate is 0% to 14% [23]. Although ATC accounts for only 2% to 5% of all thyroid cancers, it contributes more than 50% of the mortality associated with thyroid cancers [45]. When ATC is diagnosed initially, regional or distance spread is apparent in 90% of cases [6]. Fifteen percent to 50% of patients are found to have metastatic lesions at the time of diagnosis, most frequently in the lungs or bone [6789]. Although treatments of ATC with surgery, radiotherapy, and chemotherapy, alone or in combination, are conducted in many cases, there are little or no effect on patient's survival [10].
Recently, several studies demonstrated that macrophage densities in tumor microenvironment, especially M2-macrophages, were related to the poor clinical outcomes. Macrophages constitute a major type of stromal cells in tumor microenvironment. Macrophages undergo two different polarization states. In contrast to M1 macrophages, which play a role in microbicidal and tumorcidal, M2 macrophages perform immunosuppression and facilitate tumor suppression [1112]. The tumor associated macrophages (TAMs) including M2 macrophages play a key role during tumor progression and TAMs emerge as a potentially useful prognostic marker [13]. Several studies demonstrated that the high density of TAMs in cancer tissues was associated with advanced stage of cancers or poor clinical outcomes in various human cancers in such as lungs, liver, and thyroid. Recently, there were several efforts to inhibit TAMs recruitments into the tumor tissues as therapeutic strategies for cancer treatment.
Chemokines are small secretory proteins that were reported to not only regulate some important feature of cancer cells, but are also involved in the regulation of tumor angiogenesis and leukocyte recruitment [14]. Especially, CXC chemokine receptor 4 (CXCR4) is one of the most important chemokine receptors for cancer cell. CXCR4 is expressed in epithelial cancer cells and has a critical role in migration and metastasis [1516]. Lines of evidence demonstrated that overexpression of CXCR4 is a reliable indicator of metastatic potential or aggressive behavior in thyroid cancer. Additionally, CXCR4 has been shown to play a role constructing tumor microenvironment by facilitating recruitment of endothelial cells or macrophages.
The aims of this study were to investigate the clinical characteristics of ATC patients according to the TAM numbers in the tumor tissue, and to evaluate the associations between CXCR4 expressions and macrophage densities in ATC tumor microenvironment.
Thyroid tissue microarrays were constructed as previously reported [17]. Briefly, human thyroid tissues were obtained from January 1993 to December 2003 surgical pathology files from Departments of Pathology at Seoul Metropolitan Government Seoul National University Boramae Medical Center and Seoul National University Hospital. Hematoxylin and eosin (H&E) stained thyroid tissues were examined by two different pathologists with slides, and a single, appropriate paraffin block (donor block) was selected for each case. Core thyroid tissue biopsies (2 mm in diameter) were taken from individual paraffin-embedded thyroid tissue donor blocks. The final four tissue arrays consisted of samples from patients with various thyroid tissues including 15 ATC samples. All experimental procedures were done in accordance with the guidelines proposed in The Declaration of Helsinki (http://www.wma.net) involving humans. Moreover, all experiments were approved by the Institutional Review Boards of Seoul National University Hospital (1107-060-369) and Seoul Metropolitan Government Seoul National University Boramae Medical Center (06-2010-176).
Immunohistochemical analyze for CD163 (Cell Signaling, Danvers, MA, USA; diluted 1:1,000), and CXCR4 (R&D system, Minneapolis, MN, USA; diluted 1:1,000) were done on formalin-fixed, paraffin-embedded tissue sections with the BenchMark XT automated immunohistochemistry slide staining system (Ventana, Tucson, AZ, USA), according to the manufacturer's instructions. The core areas of tumors were divided into quarters, and five areas were randomly chosen from each quarter and central area and digital images were captured at 200x magnification. To evaluate the immunoreactivities of CD163+ or CXCR4+ cells, images were analyzed by ImmunoRatio [18] and percentage of positively stained nuclear area were demonstrated. The macrophage densities were defined as the fractional area of CD163+ cells per whole tumor tissue area including intracellular contents. The immunostaining intensity of CD163 or CXCL4 was further graded into two groups by median values of percentage of positively stained nuclear area: low expression and high expression groups.
To analyze of continuous variables, means and standard errors were calculated. After the distribution normality was determined, the Mann-Whitney U test was applied to the data. For analysis of the categorical variables, frequencies, and percentages were determined. Proportions were compared with the chi-square test or Fisher exact test. For survival analysis, log-rank test was performed. Statistical analysis was done with SPSS version 19.0 (IBM Co., Armonk, NY, USA). All P values were two-sided, and a P<0.05 was considered statistically significant.
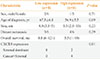
Table 1 shows the clinical characteristics of 14 ATC patients (11 women and three men). The mean diagnostic age was 65±7 years. The median tumor size was 4.3 cm ranging 2.5 to 15 cm and half of them had distant metastasis including lungs and liver at the time of diagnosis. Adjuvant therapy was conducted 10 of 14 patients, nine patients underwent external beam radiation therapy and one patient underwent concomitant chemotherapy and radiotherapy. The median overall survival was 7 months (range, 1 to 121) and all patients eventually died.
To evaluate the association between CD163-positive TAM density and clinical characteristics in human ATC, we performed immunohistochemical staining using anti-CD163 antibody and divided into two groups according to the CD163 immunoreactivities. On the basis of the median percentage of positively stained nuclear area (47.2%), eight patients belong to the low expression group and six patients to the high expression group. Because of the limited sample size, clinical characteristics were not significantly different between groups (Table 2). However, the mean diagnostic age was younger in high expression group than low expression group with marginal significance (56.9±5.5 years vs. 67.5±6.8 years, P=0.09). The median tumor size was 4.0 cm (range, 3.5 to 5.0) in low expression group and 5.3 cm (range, 2.5 to 15.0) in high expression group. Distant metastasis was observed in three of eight patients in low expression group and four of six patients in high expression group.
To further characterize TAMs in the ATC tumor microenvironment, CXCR4, a representative chemotactic signaling molecule, was stained. Fig. 2A showed representative images of CXCR4 staining in CD163-low or -high expression groups. Four patients from high expression group (n=6) showed both cancer and stromal compartments expressions of CXCR4, while all low expression group (n=8) showed stromal-dominant expressions of CXCR4, especially at perivascular area (Table 2). Additionally, regression analysis showed that the densities of CD163 and CXCR4 showed positive correlations (γ2=0.432, P=0.013) (Fig. 2B).
The present study demonstrated that high-CD163 group was significantly associated with poor overall survival in human ATC. It also showed younger diagnostic age and, larger tumor size compared to the low-CD163 group, while statistical significance was not obtained because of the small sample size. CD163 and CXCR4 expression showed strong positive correlations in ATC tissues. In addition, CXCR4 staining in high-CD163 group showed both cancer and stromal compartments expressions, whereas all low expression patients showed stromal-dominant expressions of CXCR4.
In the previous studies, high density of TAMs is present in the more advanced stage of cancers that have poor prognosis, such as beast, lung, thyroid, and bladder cancers [19202122]. Especially in thyroid cancer, Ryder et al. [23] reported that an increased density of TAMs was associated with tumor progression in advanced thyroid cancers. Qing et al. [24] found that overall density of TAMs was significantly higher in papillary thyroid cancer than benign lesions and positive associated with lymph node metastasis and TNM stage. Consistent to the previous studies, even with the limited sample size, the present study demonstrated that the high density of TAMs was associated with a shorter overall survival with in the most dedifferentiated pathologic subtype of thyroid cancer, ATC, compared to the previous studies.
ATC is one of the most aggressive human cancers with poor survival [25]. In previous study, Jung et al. [26] reported that CD163-positive macrophage density and 5-year survival rate had inverse correlations in 14 different types of human cancers, including lung, liver, pancreas, and thyroid, and ATC had the highest CD163-positive macrophages density with the lowest survival rate. Therefore, TAMs might be a potential therapeutic target for cancer treatment strategy in ATC. Indeed, several different experimental approaches have been tried for targeting TAMs: (1) inducing TAMs apoptosis using clodronate loaded liposome [2728] or zoledronic acid [2930]; (2) inhibiting monocyte differentiation into M2-macrophages using colony stimulating factor 1 receptor inhibitor [31]; and (3) stimulating repolarization into M1-macrophages using pantoprazole [32] or IL-12 [33]. Recently, Passaro et al. [34] demonstrated that oncolytic adenovirus dl922-947 reduced tumor growth and induced repolarization of TAMs into cytotoxic M1-marophages. However, whether TAMs infiltrations directly contribute to tumor growth or invasiveness or not is still elusive, and there are few studies about the characteristics of TAMs in ATC.
In order to evaluate whether TAMs, especially M2-macrophages, were accumulated in tumor microenvironments or not, we performed immunohistochemical staining using an anti-CD163 antibody, a specific maker for M2-macrophages [35]. Interestingly, in this study, CXCR4 expression was higher in the high CD163-positive TAM density group showing the co-expressions at both cancer and stromal compartments. On the other hand, low CD163-positive TAM density group showed stromal-dominant expressions of CXCR4. Since the abundance of CXCR4-positive cancer cells reflected relatively higher concentrations of chemokine 12 (CXCL12) at that microenvironment, it is reasonable to deduce that these CXCL12-rich environment enhanced macrophage infiltrations and M2-macrophages polarization. A study of breast cancer suggested that the high level of CXCL12 has led to enhance macrophages density in tumor parenchyma [36]. However, we did not evaluate the CXCL12 status in this study. Further study is needed to validate it.
One of the limitations of this study is that we cannot reveal the cellular origin of the stromal expression of CXCR4. CXCR4 is widely expressed in various cells including most leukocytes, macrophages, mesenchymal stromal cells, and endothelial cells [37383940], since CD163 and CXCR4 expression had a positive correlations, we could postulate that a part of CXCR4-positive stromal compartments were macrophages, or CXCR4-positive stromal compartments contributed to macrophages recruitment. Further experimental study is needed to reveal the causal relationship and the concrete cellular interactions.
In conclusion, high density of CD163-positive TAMs showed poor overall survival with aggressive clinical characteristics in ATC, suggesting the potential of TAMs as a prognostic biomarker for ATC. Furthermore, CXCR4 expression was significantly correlated with CD163-positive TAM densities, which suggest the possible role of CXCR4 in TAM recruitments. To validate the potentials of CXCR4 and TAMs as therapeutic targets of ATC treatment, further studies are needed to understand the molecular mechanisms of TAMs recruitment in relationship with CXCR4.
Figures and Tables
Fig. 1
Kaplan-Meier curves for overall survival of anaplastic thyroid cancer patients groups according to tumor-associated macrophages density.

Fig. 2
Expressions of CXC chemokine receptor 4 (CXCR4) and CD163 in anaplastic thyroid cancer tissues. (A) Represent immunohistochemical staining images of CD163 and CXCR4. (B) Correlations between CD163 and CXCR4 expressions. DAB, 3,3'-diaminobenzidine.

Table 1
Clinicopathological Characteristics of Anaplastic Thyroid Cancer Patients

Table 2
Clinicopathological Characteristics according to the CD163 Expressions
ACKNOWLEDGMENTS
This work was supported by a research grant from National Medical Center (No.NMC2014-MS-04) and Korean Endocrine Society.
References
1. Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005; 103:1330–1335.
2. Brignardello E, Gallo M, Baldi I, Palestini N, Piovesan A, Grossi E, et al. Anaplastic thyroid carcinoma: clinical outcome of 30 consecutive patients referred to a single institution in the past 5 years. Eur J Endocrinol. 2007; 156:425–430.
3. Lo TE, Jimeno CA, Paz-Pacheco E. Anaplastic thyroid cancer: experience of the Philippine General Hospital. Endocrinol Metab (Seoul). 2015; 30:195–200.
4. Giuffrida D, Gharib H. Anaplastic thyroid carcinoma: current diagnosis and treatment. Ann Oncol. 2000; 11:1083–1089.
5. Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol. 2011; 2011:542358.
6. McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001; 130:1028–1034.
7. Nel CJ, van Heerden JA, Goellner JR, Gharib H, McConahey WM, Taylor WF, et al. Anaplastic carcinoma of the thyroid: a clinicopathologic study of 82 cases. Mayo Clin Proc. 1985; 60:51–58.
8. Carcangiu ML, Steeper T, Zampi G, Rosai J. Anaplastic thyroid carcinoma. A study of 70 cases. Am J Clin Pathol. 1985; 83:135–158.
9. Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990; 66:321–330.
10. Veness MJ, Porter GS, Morgan GJ. Anaplastic thyroid carcinoma: dismal outcome despite current treatment approach. ANZ J Surg. 2004; 74:559–562.
11. Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008; 66:1–9.
12. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009; 86:1065–1073.
13. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006; 66:605–612.
14. Zlotnik A. Chemokines and cancer. Int J Cancer. 2006; 119:2026–2029.
15. Kim CH, Broxmeyer HE. SLC/exodus2/6Ckine/TCA4 induces chemotaxis of hematopoietic progenitor cells: differential activity of ligands of CCR7, CXCR3, or CXCR4 in chemotaxis vs. suppression of progenitor proliferation. J Leukoc Biol. 1999; 66:455–461.
16. Aiuti A, Tavian M, Cipponi A, Ficara F, Zappone E, Hoxie J, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999; 29:1823–1831.
17. Cho SW, Kim YA, Sun HJ, Ahn HY, Lee EK, Yi KH, et al. Therapeutic potential of Dickkopf-1 in wild-type BRAF papillary thyroid cancer via regulation of β-catenin/E-cadherin signaling. J Clin Endocrinol Metab. 2014; 99:E1641–E1649.
18. Tuominen VJ, Ruotoistenmaki S, Viitanen A, Jumppanen M, Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 2010; 12:R56.
19. Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011; 128:703–711.
20. Kim S, Cho SW, Min HS, Kim KM, Yeom GJ, Kim EY, et al. The expression of tumor-associated macrophages in papillary thyroid carcinoma. Endocrinol Metab (Seoul). 2013; 28:192–198.
21. Sato S, Hanibuchi M, Kuramoto T, Yamamori N, Goto H, Ogawa H, et al. Macrophage stimulating protein promotes liver metastases of small cell lung cancer cells by affecting the organ microenvironment. Clin Exp Metastasis. 2013; 30:333–344.
22. Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012; 7:e50946.
23. Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008; 15:1069–1074.
24. Qing W, Fang WY, Ye L, Shen LY, Zhang XF, Fei XC, et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid. 2012; 22:905–910.
25. Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006; 13:453–464.
26. Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, et al. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med. 2015; 49:318–324.
27. Piaggio F, Kondylis V, Pastorino F, Di Paolo D, Perri P, Cossu I, et al. A novel liposomal clodronate depletes tumor-associated macrophages in primary and metastatic melanoma: anti-angiogenic and anti-tumor effects. J Control Release. 2016; 223:165–177.
28. Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006; 95:272–281.
29. Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med. 2011; 9:177.
30. Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Hegmans JP, et al. Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma. Br J Cancer. 2010; 103:629–641.
31. Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci U S A. 2005; 102:16078–16083.
32. Vishvakarma NK, Singh SM. Immunopotentiating effect of proton pump inhibitor pantoprazole in a lymphoma-bearing murine host: implication in antitumor activation of tumor-associated macrophages. Immunol Lett. 2010; 134:83–92.
33. Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007; 178:1357–1362.
34. Passaro C, Borriello F, Vastolo V, Di Somma S, Scamardella E, Gigantino V, et al. The oncolytic virus dl922-947 reduces IL-8/CXCL8 and MCP-1/CCL2 expression and impairs angiogenesis and macrophage infiltration in anaplastic thyroid carcinoma. Oncotarget. 2016; 7:1500–1515.
35. Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012; 375:196–206.
36. Boimel PJ, Smirnova T, Zhou ZN, Wyckoff J, Park H, Coniglio SJ, et al. Contribution of CXCL12 secretion to invasion of breast cancer cells. Breast Cancer Res. 2012; 14:R23.
37. Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, et al. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005; 97:1142–1151.
38. Schramm B, Penn ML, Speck RF, Chan SY, De Clercq E, Schols D, et al. Viral entry through CXCR4 is a pathogenic factor and therapeutic target in human immunodeficiency virus type 1 disease. J Virol. 2000; 74:184–192.
39. Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999; 283:845–848.
40. Mohle R, Schittenhelm M, Failenschmid C, Bautz F, Kratz-Albers K, Serve H, et al. Functional response of leukaemic blasts to stromal cell-derived factor-1 correlates with preferential expression of the chemokine receptor CXCR4 in acute myelomonocytic and lymphoblastic leukaemia. Br J Haematol. 2000; 110:563–572.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download