Abstract
Background
Hyponatremia developing in hypothyroid patients has been encountered in clinical practice; however, its prevalence has not been well established.
Methods
Thirty patients diagnosed with differentiated thyroid cancer, rendered hypothyroid after surgery and levothyroxine withdrawal, and who are for radioactive iodine (RAI) ablation were included. Serum sodium concentrations were measured twice, at the time of admission for RAI ablation, and before discharge after increased oral fluid intake. The outcome measures were to determine the prevalence of hyponatremia among hypothyroid patients prior to RAI ablation and after oral hydration post-RAI, and to correlate the serum sodium levels pre-RAI and post-RAI with thyroid-stimulating hormone (TSH) concentration and age.
Results
Thirty patients were included, with ages from 23 to 65 years old (median, 40). Two patients (6.7%) were hyponatremic prior to RAI ablation, and eight patients (26.7%) had mild hyponatremia (130 to 134 mEq/L) after RAI and hydration. There was no significant correlation between TSH levels and serum sodium levels prior to or after RAI. There was also no significant correlation between pre- and post-RAI sodium concentration and age.
Conclusions
The prevalence of hyponatremia pre-RAI was 6.7%, and 26.7% post-RAI. No significant correlation was noted between TSH concentration and age on pre- or post-RAI sodium concentrations. Routine measurement of serum sodium post-RAI/isolation is still not advised. Measurement of sodium post-RAI may be considered in patients who are elderly, with comorbid conditions or on medications.
Hyponatremia in hypothyroidism is commonly seen in clinical practice. According to Baajafer et al. [1], the prevalence of mild hyponatremia (serum sodium <135 mEq/L) on short-term uncomplicated hypothyroidism has been noted at 3.9%. The exact mechanism for the development of hyponatremia in hypothyroidism has not been well-established; some theories postulate that decreased water clearance and inappropriate concentrations of anti-diuretic hormone (ADH) levels may be involved in developing dilutional hyponatremia [2].
In the management of patients with differentiated thyroid cancer, although surgery is the primary therapy, radioactive iodine (RAI) therapy is warranted after total thyroidectomy, especially in high-risk patients. RAI is necessary for ablation of residual thyroid tissue as well as ablation of residual tumor and metastatic disease [2]. In preparation for radioiodine therapy, patients are allowed to be hypothyroid (thyroid-stimulating hormone [TSH] >25 mIU/L), expectantly seen in post-thyroid-ectomy patients, for better uptake during RAI. Thyroid hormone treatment is withdrawn and patients are instructed to maintain a low iodine diet, involving restricted salt and increased fluid intake. In a case series done by Nozu et al. [3] involving patients with thyroid cancer, seven cases of symptomatic hyponatremia were associated with 131I preparation-induced hypothyroidism. This occurred at approximately 4 weeks after thyroid hormone withdrawal and 2 weeks after low iodine diet, respectively. The incidence and magnitude of hyponatremia, the severity of hypothyroidism, old age (more than 60 years old) proposed as risk factors for developing hyponatremia after RAI therapy [45]. Case reports of life-threatening hyponatremia developing in hypothyroid patients have also been reported, either due to post-surgical hypothyroidism or levothyroxine withdrawal; however, its prevalence has not been well established in this population [6]. In current practice, serum sodium levels are not in these patients prior to RAI therapy or on discharge after RAI treatment. With several reports of unforeseen life-threatening complications in this population, such as vomiting, confusion, hallucination, seizure, coma, and death, the need to investigate the degree of hyponatremia is realized. This study aimed to determine the prevalence of hyponatremia in hypothyroid patients pre- and post-RAI. Secondary objectives were aimed to describe the demographic profile of hypothyroid cancer patients, to determine the correlation of TSH levels with serum sodium levels pre- and post-RAI, and to determine the correlation of age and serum sodium levels pre- and post-RAI.
A prospective cohort study design was used to descibe the prevalence of hyponatremia in hypothyroid patients with differentiated thyroid cancer who received RAI. Serum sodium levels were assessed prior to radioactive therapy and was followed over a period of time. Reassessment of serum sodium levels was done post RAI.
The study recruited patients at University of Santo Tomas Hospital from July 2014 to December 2015. This was done after obtaining approval from the Hospital Institutional Review Board and Ethics Committee. All patients signed an informed consent.
Participants of this study were a random sample of patients from the clinical and private out-patient department. All patients diagnosed with differentiated thyroid cancer and who had undergone total or near-total thyroidectomy, regardless of age and gender, and were admitted for RAI treatment were included. Subjects who had consented to be enrolled in the study underwent a short history and physical examination. To minimize possible bias, patients with comorbid conditions (heart failure, acute and chronic kidney disease, and liver cirrhosis), with intake of medications such as diuretics and lithium, and with active infections (viral or bacterial) were excluded from the study. TSH assay was performed prior to RAI. A 3 mL of blood was extracted from each subject by venipuncture, and sent to the hospital laboratory for determination of TSH using ultrasensitive assay (immunoradiometric assay [IRMA], Isotopes Ltd., Budapest, Hungary). The normal range for TSH is 0.27 to 4.2 U/L; only patients with elevated TSH (≥25 U/L) were included in the study. Participants were followed-up and observed until immediately after post-RAI therapy.
Sample size calculation was based on the formula for estimation of proportion (n=Z2PQ/d2). The prevalence data was based on a previous retrospective study done by Baajafer et al. [1] in 1999 on short-term uncomplicated hypothyroidism. It included 24 patients with only 3.9% prevalence of hyponatremia. The sample size computed was 59, which was an estimate allowing 10% error at 95% confidence interval.
where Z=reliability level and power of the test
P=proportion
Q=(1–P)
d=percent allowable error
N=1.962(0.04)(0.96)/(0.05)2=59
Levothyroxine treatment was withdrawn for 4 weeks during which patients were instructed to follow a strict low-iodine, low-salt diet.
Serum sodium concentrations were measured twice, at the time of admission for RAI ablation, and on the day of discharge (2 to 3 days after RAI ablation), after hydration with 3 to 4 L of water per day. This was done by obtaining 3 mL of blood by venipuncture for each variable measured. The primary end-point measures were serum sodium concentrations before admission and on discharge. Mean sodium concentrations and the prevalence of mild hyponatremia (130 to 135 mEq/L), moderate hyponatremia (120 to 129 mEq/L), and severe hyponatremia (<120 mEq/L) were determined [3]. The normal reference range for serum sodium concentrations is 136 to 146 mEq/L. Clinical symptoms of hyponatremia (such as headache, confusion, seizures, weakness, muscle fatigue, anorexia, nausea, vomiting, diarrhea, hypertension or bradycardia, oliguria) was observed. Clinical assessments were done by the physician on admission. All procedures were done similarly on all patients.
Descriptive analysis of the baseline characteristics of the hypothyroid patients were generated. Mean, standard deviation, and range were used to describe continuous variables (serum sodium levels). Median and interquartile range (IQR) were used to describe non-parametric variables (age and TSH concentration). Sex was described using frequency and percentage. Correlations were analyzed using Pearson r test. Pre- and post-RAI serum sodium levels were compared with age, sex, and TSH using Fisher exact test for continuous variables and chi-square for categorical variables. Subgroup analysis of the age group (<40 or ≥40) and sex were generated to determine if these factors affected the serum sodium levels of hypothyroid patients post-RAI. A P value of less than 0.05 was statistically significant.
Of the 36 patients that were initially sampled for the study, six patients were excluded due to intake of medications (four on lithium and two on hydrochlorothiazides), leaving 30 hypothyroid patients diagnosed with differentiated thyroid cancer who were included and prospectively studied. Twenty-four (80%) were females. Ages ranged from 23 to 65 years old, with median age of 40 (IQR, 18). TSH concentrations at baseline ranged from 26.7 to >100 U/L. Three of the patients had hypertension but were not maintained on diuretics or aldosterone receptor blockers. Data of the 30 patients is summarized (Table 1). None of the participants had missing data for each variable of interest. During the approximate 4 to 5 days duration of RAI therapy, the patients were also observed for possible signs and symptoms of hyponatremia.
Prior to RAI, one patient (3.3%) had moderate hyponatremia (129 mEq/L), while one patient (3.3%) had mild hyponatremia (133 mEq/L) (Table 2). Mean pre-RAI sodium concentration was 139 mEq/L. After RAI, mild hyponatremia was present in eight patients (26.7%) and moderate/severe hyponatremia was not observed in any of the patients. There was a significant correlation between pre- and post-RAI sodium concentrations with r=0.776 (P<0.001). Non-specific symptoms, namely muscle weakness and headache were observed in six (20%) of the patients that were admitted. Of all these six patients, serum sodium levels were more than 135 mEq/L.
Regarding TSH concentrations and hyponatremia, there was no significant correlation between pre-RAI sodium concentration and TSH concentration (r=0.055, P=0.775) or between post-RAI sodium concentrations and TSH concentration (r=–0.159, P=0.401). There was also no significant correlation between pre- and post-RAI sodium concentration and age (r=0.028, P=0.88 and r=–0.062, P=0.75, respectively) (Table 3).
Subgroup analysis of pre- and post-RAI serum sodium levels according to age <40 or ≥40 years showed no significant association between age and hyponatremia both in pre- and post-RAI with P value 0.58 and 0.60, respectively (Table 4). No significant difference was noted between males and females and the development of hyponatremia pre- and post-RAI (Table 4).
In this study, we hypothesized that degrees of hyponatremia could be present in hypothyroid patients; however, its frequency and severity has not been well documented. In our present study of 30 patients with hypothyroidism, hyponatremia after RAI and isolation was fairly common at a prevalence of 26.7%; however, there was still no noted statistical significance with the correlation of degree of hyponatremia with that of age and levels of TSH.
Hyponatremia secondary to hypothyroidism may be due to several mechanisms involving renal, cardiovascular and hypothalamo-adrenal systems. In hypothyroidism, there is decrease in free water formation and urine volume [7]. The mechanism of diminished water excretion is increased plasma ADH promoting water retention. In a study by Ota et al. [7], patients with severe hypothyroidism had increased plasma ADH but it did not decrease in response to a fall in plasma osmolality after an acute water load. This suggests that there is impaired osmoregulation of ADH release and/or a decrease in the metabolic clearance rate of ADH.
Renal hypofunction characterized by reductions in renal glomerular filtration rate and renal blood flow is well documented in hypothyroidism [8]. This contributes further to a fall in free water formation resulting in hyponatremia. Whether or not they have hyponatremia, patients with hypothyroidism have a diminished ability to excrete free water, impaired maximum urinary dilution and delayed excretion of water load.
Hypothyroidism also affects most components of the renin-angiotensin-aldosterone system, including the secretion of renin, the hepatic production of angiotensinogen, the adrenal production and metabolism of aldosterone. The decrease in the hepatic clearance of aldosterone results in an increase in its plasma half-life [9]. However, results of a study done by Koide et al. [10] showed that neither vasopressin nor aldosterone plays a dominant role in the pathogenesis of the hyponatremia in patients with hypothyroidism. It appears that thyroid-hormone deficiency itself caused the derangement of tubular-cell function, which resulted in the development of the impaired water excretion and hyponatremia.
In preparation for RAI ablation, patients are advised to maintain a low iodine diet, and thyroid hormone replacement is usually withdrawn 4 to 6 weeks prior to RAI. This preparation renders them hypothyroid, which increases endogenous levels of TSH and subsequently stimulates the uptake of 131I in remnant thyroid cells as well as in differentiated thyroid cancer cells. However, this induced temporary hypothyroidism has been shown to cause substantial morbidities such as cold intolerance, periorbital puffiness, weight gain, and constipation [11]. Case reports of 10 elderly patients have also demonstrated development of hyponatremia during preparation for RAI. Data analyzed from these reports suggested that the development of hyponatremia in these patients was related to age (>65 years), concomitant diuretic therapy, and low iodine diet. Low-iodine diet results in low overall solute intake, which strongly contributes to the development of hyponatremia in this population of patients in which renal impairment is common [12].
With the advent of exogenously administered recombinant human TSH (rhTSH), patients can remain on thyroid replacement; thereby, avoiding the associated morbidity of hypothyroidism. Based on a systemic review last 2009 [11], the efficacy of rhTSH in the preparation of patients for RAI was found to be equivalent to the traditional method of thyroid hormone withdrawal. Thus, the American Thyroid Association released guidelines on the management of patients with differentiated thyroid cancer, which issued a level B recommendation on the use of either thyroid hormone withdrawal or rhTSH for radioiodine remnant ablation [11]. Therefore, in patients who are unable to tolerate prolonged hypothyroidism or are unable to achieve satisfactory elevation of endogenous TSH by means of thyroid hormone withdrawal, rhTSH may be the only option for RAI; however, in our local setting wherein rhTSH is not commercially available, this option remains non-viable.
In a case report by Sari and Sevinc [6], severe euvolemic hyponatremia developed in a patient with hypothyroidism who did not take the recommended L-thyroxine treatment. Another case reported by Nozu et al. [3] cited a case of severe hyponatremia at 98 mEq/L which occurred 12 days after restarting thyroid hormone replacement and cessation of diet restriction. This suggests that hyponatremia may occur or persist days after RAI therapy. Whatever the mechanism of hyponatremia in cases of hypothyroidism, it should be noted that severe hyponatremia may occur in patients with hypothyroidism due to either irregular levothyroxine usage or cessation of treatment.
The frequency and severity of hyponatremia in hypothyroid patients without comorbid conditions have not been well documented. A retrospective study done on short-term uncomplicated hypothyroidism had a prevalence of 3.9% with serum sodium of <135 mEq/L [1]. A prospective study done by Hammami et al. [2] done on 212 patients with differentiated thyroid cancer showed development of hyponatremia after RAI and isolation in 10.4% of the patients. It was concluded that clinically-important hyponatremia was uncommon in this setting. However, in our present study of 30 patients with hypothyroidism (TSH >25 mIU/L), hyponatremia after RAI and isolation was fairly common at a prevalence of 26.7% while it was noted to be 6.7% pre-RAI. Contributory factors that could explain this include nausea that may be experienced after RAI treatment, as well as the increase in patient's anxiety associated with isolation after RAI treatment, which are potent stimulators of ADH secretion. Therefore, increased water intake, nausea, and anxiety that is associated after RAI treatment/isolation may lead to clinically significant hyponatremia [2]. However, despite the relatively high prevalence of post-RAI hyponatremia, there was still no significant correlation noted between post-RAI sodium concentrations and TSH levels. This is consistent with a previous study done by Hammami et al. [2] and Lee et al. [4], wherein they also noted no significant correlation between post-isolation sodium concentration and TSH levels. It was concluded in the previous studies that the association between clinically-significant hyponatremia and hypothyroidism may be due to restricted to chronic uncorrected hypothyroidism [2]. Since our data showed that hypothyroidism (elevated TSH >25) is not significantly correlated with pre- or post-RAI hyponatremia, these suggest that other risk factors (namely age and comorbids) may be associated with the development of hyponatremia after RAI therapy.
Despite the noted high percentage of patients who developed hyponatremia, it is notable that none of the participants developed specific symptoms of hyponatremia. This may be due to the fact that the lowest sodium level noted in our study was only129 mEq/L. In the case report of Nozu et al. [3], symptoms (such as lethargy, nausea, and general fatigue) were only noted at severe hyponatremic levels of 110 to 121 mEq/L. Thus, clinically significant and life-threatening complications are more notable at moderate to severe degrees of hyponatremia.
In our study, there was a significant difference (P<0.001) or drop between pre- and post-RAI sodium concentrations among all the participants; however, in subgroup comparison between males and females, there was no noted significant difference in the drop of serum sodium concentration pre- and post-RAI. These results are consistent with the findings of some studies, in which it was stated that there is no gender preference in the development of hyponatremia for females [13].
Some studies have noted that age is an independent risk factor for hyponatremia [13]. In a study of Lee et al. [4], old age, namely >60 years old, was shown to be a risk factor for developing hyponatremia after RAI therapy. It was postulated that there is delay in the ability of the senile kidney to lower sodium excretion in a hyponatremic state. Compared to younger individuals, elderly patients in a hypothyroid state are more susceptible to hyponatremia because of excessive ADH. However, this was not demonstrated in our present study. There was no significant correlation between age and pre- or post-RAI sodium concentrations. This may be due to the fact that the median age of the study group was 40, and only four subjects were >60 years old.
Limitation of this study was the small sample size, wherein our figures may overestimate the results of this study. Other comorbid conditions that are not known both to the subject and principal investigator at the time of examination may also affect the study. Likewise, patient related factors such as the amount of hydration done post-RAI, and adherence to low salt, low iodine pre-RAI therapy may also contribute to the results of this study.
In our study, the prevalence of hyponatremia pre-RAI was 6.7%, while the prevalence post-RAI was high at 26.7%. No significant correlation was noted between TSH concentration, age and sex on pre- or post-RAI serum sodium concentration.
With the noted high prevalence of hyponatremia post-RAI, we can be more vigilant to consider measurement of serum sodium levels pre- and post-RAI in patients with the following risk factors: elderly, patients on medications, and patients with comorbidities such heart failure, cirrhosis, and renal failure. Physicians should be aware and educated on the development of this complication, as well as the appropriate management of such cases. Several factors also have to be taken into account, including patient's renal status, comorbid conditions, and intake of medications such as diuretics.
Figures and Tables
Table 1
Baseline Characteristics of Hypothyroid Patients (n=30)

Table 2
Frequency Distribution of Hyponatremia Pre-RAI and Post-RAI (n=30)

| Characteristic | No. (%) |
|---|---|
| Pre-RAI | |
| Mild hyponatremia | 1 (3.33) |
| Moderate hyponatremia | 1 (3.33) |
| Post-RAI | |
| Mild hyponatremia | 8 (26.67) |
Table 3
Correlation of Age and TSH Levels with Serum Sodium Levels Pre-RAI and Post-RAI (n=30)

| Characteristic | Pre-RAI serum Na level | Post-RAI serum Na level | ||
|---|---|---|---|---|
| Pearson correlation (r) | P value | Pearson correlation (r) | P value | |
| Age | 0.028 | 0.883 | −0.062 | 0.746 |
| TSH concentration | 0.055 | 0.775 | −0.159 | 0.401 |
Table 4
Age and Sex Subgroup Analysis of Hyponatremia Pre-RAI and Post-RAI
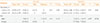
Values are expressed as number (%). Pearson chi-square test assumption not passed.
RAI, radioactive iodine; SD, standard deviation.
aFour cells (66.7%) have expected count less than 5. The minimum expected count is 0.20; bTwo cells (50.0%) have expected count less than 5. The minimum expected count is 1.60.
References
1. Baajafer FS, Hammami MM, Mohamed GE. Prevalence and severity of hyponatremia and hypercreatininemia in short-term uncomplicated hypothyroidism. J Endocrinol Invest. 1999; 22:35–39.
2. Hammami MM, Almogbel F, Hammami S, Faifi J, Alqahtani A, Hashem W. Acute severe hypothyroidism is not associated with hyponatremia even with increased water intake: a prospective study in thyroid cancer patients. BMC Endocr Disord. 2013; 13:27.
3. Nozu T, Yoshida Y, Ohira M, Okumura T. Severe hyponatremia in association with I(131) therapy in a patient with metastatic thyroid cancer. Intern Med. 2011; 50:2169–2174.
4. Lee JE, Kim SK, Han KH, Cho MO, Yun GY, Kim KH, et al. Risk factors for developing hyponatremia in thyroid cancer patients undergoing radioactive iodine therapy. PLoS One. 2014; 9:e106840.
5. Werner S, Ingbar SH, Braverman L, Utiger RD. The thyroid: a fundamental and clinical text. 9th ed. Philadelphia: Lippincott Williams & Wilkins;2005. p. 791.
6. Sari R, Sevinc A. Life-threatening hyponatremia due to cessation of L-thyroxine. J Natl Med Assoc. 2003; 95:991–994.
7. Ota K, Kimura T, Sakurada T, Shoji M, Inoue M, Sato K, et al. Effects of an acute water load on plasma ANP and AVP, and renal water handling in hypothyroidism: comparison of before and after L-thyroxine treatment. Endocr J. 1994; 41:99–105.
8. Montenegro J, Gonzalez O, Saracho R, Aguirre R, Gonzalez O, Martinez I. Changes in renal function in primary hypothyroidism. Am J Kidney Dis. 1996; 27:195–198.
9. Werner SC, Ingbar SH, Braverman LE, Utiger RD. Chapter 61, The kidney and electrolyte metabolism in hypothyroidism. Werner & Ingbar's the thyroid: a fundamental and clinical text. 8th ed. Philadelphia: Lippincott Williams & Wilkins;2000. p. 791–792.
10. Koide Y, Oda K, Shimizu K, Shimizu A, Nabeshima I, Kimura S, et al. Hyponatremia without inappropriate secretion of vasopressin in a case of myxedema coma. Endocrinol Jpn. 1982; 29:363–368.
11. Yoo J, Cosby R, Driedger A. Preparation with recombinant humanized thyroid- stimulating hormone before radioiodine ablation after thyroidectomy: a systematic review. Curr Oncol. 2009; 16:23–31.
12. Pantalone KM, Hatipoglu BA. Hyponatremia and the thyroid: causality or association? J Clin Med. 2015; 4:32–36.
13. Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003; 337:169–172.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


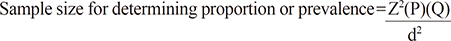
 XML Download
XML Download