Abstract
Management of diabetic complications has been a worldwide major global health issue for decades. Recent studies from many parts of the world indicate improvement in this area. However, it is unknown if such an improvement is being realized in Koreans. Although there is limited information regarding diabetic retinopathy management among Koreans, recent epidemiologic studies have indicated improved screening rates and less frequent visual impairment among type 2 diabetics. Moreover, results achieved with new diagnostic and treatment modalities aimed to improve diabetic retinopathy management are encouraging for both physicians and patients.
Go to : 
Despite a rapid increase in the prevalence of diabetes worldwide, recent data from epidemiologic studies indicate that the prevalence of diabetic retinopathy (DR) is decreasing in many parts of the world [123]. Moreover, the prevalence of the severe form of DR, which leads to visual impairment, is also decreasing [4]. According to a recent report of health insurance data from Taiwan, there was a decreasing trend in the age-adjusted prevalence rates of sight-threatening DR, with a mean of 2.75% for women and 2.87% for men [5]. However, information regarding recent changes in DR prevalence or clinical practice patterns for diagnosis and treatment in Korea is scarce. To clarify this issue, we aimed to address comprehensive changes in DR epidemiology over recent years and to understand recent changes in the detection and treatment of DR in type 2 diabetes patients in Korea.
Go to : 
DR is known to be one of the major causes of vision loss worldwide [678]. As the consequence of a very considerable increase in the prevalence of diabetes in Korea, many more Koreans will suffer from DR in the near future [9]. In 1998, the incidence of DR in Korea was reported to be 44.4/1,000 people/year, though by 2013, the incidence of DR had reportedly increased to 56/1,000 people/year [1011]. However, since the study participants and follow-up periods were different, it is difficult to compare figures from these two reports directly. While the increase in incidence of DR amongst Koreans undoubtedly mirrors the rapid increase of diabetes in the general population, improvements in DR screening during the past decade have also resulted in better detection of DR (and thus a further increase in DR incidence) [12]. According to data from the National Insurance Service Survey conducted by the Korean Diabetes Association, about 98% of all diabetic patients underwent at least one dilated fundus examination in the period 2006 to 2013. In addition, 80% of patients underwent a dilated fundus examination every 2 years during this period. However, when it came to continuity of screening (defined as an annual dilated fundus examination), the proportion was much lower. Thus, only 24% of patients underwent an annual dilated fundus examination in 2006, and this increased to 30% in 2013. This level of continuity of screening was much lower in comparison to that in other countries, where continuity of screening levels of 50% have been reported [131415]. Therefore, a greater effort from diabetes care providers is necessary to improve continuity of screening for DR, which is crucial for the management of diabetes-related complications.
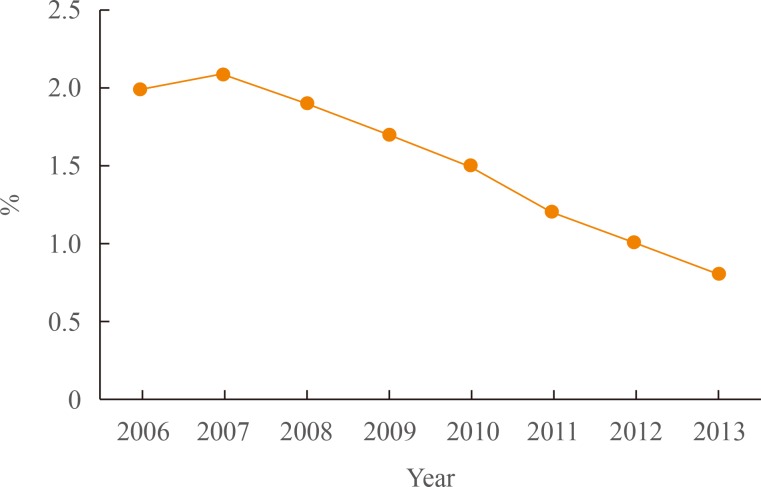
According to recent National Insurance Service Survey results, the prevalence of DR remained steady in the period between 2006 and 2013 (14% vs. 16%) [12]. However, complacence over this result would be misplaced, since the prevalence of DR may not as of yet reflect the recent rapid increase in the diabetic population during the same study period. Indeed, there may be a considerable time lag between the development or progression of DR and onset of diabetes. Longitudinal studies are required to follow the development of DR once diabetes has been diagnosed. Among patients with diabetes who had DR, the proportion of patients who had less than 0.1 best corrected visual acuity decreased from 2.0% (4,820/237,267) in 2006 to 0.8% (3,572/431,964) in 2013 (Fig. 1).
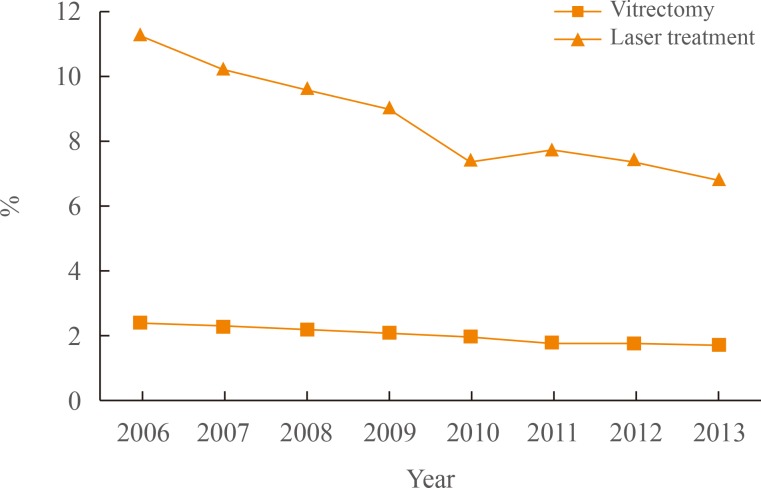
The introduction of an intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) for DR has had a huge impact on the DR treatment paradigm worldwide [16171819]. And intravitreal injection of anti-VEGF agent has now become the principal treatment modality for diabetic macular edema. A Diabetic Retinopathy Clinical Research Network (DRCR.net) study (one of several clinical studies reporting similar results) has presented evidence that eyes receiving anti-VEGF therapy for diabetic macular edema demonstrate better long-term vision improvements than eyes managed with conventional laser or combination treatments [18]. Moreover, due to the extensive use of anti-VEGF intravitreal injections, the number of patients requiring laser treatment or vitrectomy is likely to decrease substantially. According to figures provided in the National Insurance Service Survey, the number of patients who received laser treatment or underwent vitrectomy decreased during the 2006 to 2013 period (Fig. 2). The proportion of patients who received laser or vitrectomy decreased by almost half (laser, 7% vs. 11%; vitrectomy, 2% vs. 7%) during this period [12]. However, it is still too early to conclude that anti-VEGF treatment will completely replace other treatment modalities (including laser treatments). The short-term effect of anti-VEGF treatment and the need for subsequent multiple injections (which can be a substantial economic burden for DR patients) remain significant disadvantages.
Among the important advancements in DR diagnosis over the past decade, the increasing role of ultrawide field fundus imaging is noteworthy [202122]. Ultrawide field fundus imaging allows up to 200° of the peripheral retina to be captured in a single frame. Thus, ultrawide-field imaging visualizes a 3.2 times larger retinal surface area than conventional methods [202122]. Moreover, ultrawide field fundus fluorescein angiography images allow visualization of more than twice the area of nonperfusion and neovascularization in comparison with more conventional fundus fluorescein angiography [202122]. Improved visualization of the retinal periphery through ultrawide field fundus imaging may result in earlier diagnosis and better treatment results. In addition, there is the convenience of retina examinations without mandatory pupil dilation.
There have been extensive attempts to develop new diagnostic modalities to help in the early diagnosis of DR. The potential use of retinal vessel changes as a unique diagnostic marker for DR is one field of particular interest [232425]. Retinal vessel diameter has commonly been associated with various systemic pathologic vascular diseases such as stroke and ischemic heart disease [26272829]. Klein et al. [28] reported that retinal vessel caliber is independently associated with risk of incident nephropathy, lower extremity amputation, and stroke mortality in persons with type 2 diabetes. More recently, changes in retinal vessel caliber (mainly decrease of retinal vessel diameter) in eyes with diabetic macular edema following laser treatment or intravitreal injections have been reported [26]. A potential role for systematic observation of retinal vessel change to monitor treatment response for diabetic macular edema has been proposed. However, until recently, adequate information regarding the relationship between retinal vessel changes and DR has not been available. Additional studies to determine the clinical implications of retinal vessel changes regarding DR are essential.
Although recent advancements in digital technologies have helped to improve diagnostic accuracy and effectiveness in many medical fields, several such developments have helped transform the diagnosis of diabetes. In particular, telemedicine programs have the capability to distribute quality eye care to any location [30313233]. In addition, various programs have recently become available for automated or semi-automated DR detection using fundus images [282930]. Most of these programs report outstandingly high sensitivity and specificity [30313233]. Hence, automated detection of DR using fundus images may improve accessibility and cost-effectiveness for DR screening in the near future.
Go to : 
Knowledge that the majority of type 2 diabetes patients have undergone at least one dilated fundus examination during 2006 to 2013 is a relief, as is the news that visual impairment among patients with type 2 diabetes maybe decreasing. In addition, several advancements in the diagnosis and treatment of DR have been made. Nevertheless, we need to focus on improving the continuity of DR screening and to achieve this, comprehensive and continuous effort is needed from both doctors and patients.
Go to : 
ACKNOWLEDGMENTS
The authors would like to thank the Korean task force team for the diabetes fact sheet of the Korean Diabetes Association and the Korean National Health Insurance Service (NHIS) for data collection and analysis.
Go to : 
References
1. Klein R, Klein BE. Are individuals with diabetes seeing better?: a long-term epidemiological perspective. Diabetes. 2010; 59:1853–1860. PMID: 20668290.
2. Centers for Disease Control and Prevention (CDC). Self-reported visual impairment among persons with diagnosed diabetes: United States, 1997-2010. MMWR Morb Mortal Wkly Rep. 2011; 60:1549–1553. PMID: 22089967.
3. Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008; 115:1859–1868. PMID: 19068374.
4. Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003; 26:1258–1264. PMID: 12663607.

5. Lin JC, Shau WY, Lai MS. Sex- and age-specific prevalence and incidence rates of sight-threatening diabetic retinopathy in Taiwan. JAMA Ophthalmol. 2014; 132:922–928. PMID: 24809869.

6. Keeffe J, Taylor HR, Fotis K, Pesudovs K, Flaxman SR, Jonas JB, et al. Prevalence and causes of vision loss in Southeast Asia and Oceania: 1990-2010. Br J Ophthalmol. 2014; 98:586–591. PMID: 24407561.

7. Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82:844–851. PMID: 15640920.
8. Yoon KC, Mun GH, Kim SD, Kim SH, Kim CY, Park KH, et al. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008-2009. Korean J Ophthalmol. 2011; 25:421–433. PMID: 22131780.

9. Korean Diabetes Association. Health Insurance Review & Assessment Service. Report of task force team for basic statistical study of Korean diabetes mellitus: diabetes in Korea 2007. Seoul: Goldfishery;2008.
10. Kim HK, Kim CH, Kim SW, Park JY, Hong SK, Yoon YH, et al. Development and progression of diabetic retinopathy in Koreans with NIDDM. Diabetes Care. 1998; 21:134–138. PMID: 9538984.

11. Kim YJ, Kim JG, Lee JY, Lee KS, Joe SG, Park JY, et al. Development and progression of diabetic retinopathy and associated risk factors in Korean patients with type 2 diabetes: the experience of a tertiary center. J Korean Med Sci. 2014; 29:1699–1705. PMID: 25469073.

12. Korean Diabetes Association. Health Insurance Review & Assessment Service. Report of task force team for Korean diabetes fact sheet 2015. Seoul: Goldfishery;2015.
13. Sloan FA, Yashkin AP, Chen Y. Gaps in receipt of regular eye examinations among medicare beneficiaries diagnosed with diabetes or chronic eye diseases. Ophthalmology. 2014; 121:2452–2460. PMID: 25208856.

14. Wang D, Ding X, He M, Yan L, Kuang J, Geng Q, et al. Use of eye care services among diabetic patients in urban and rural China. Ophthalmology. 2010; 117:1755–1762. PMID: 20471689.

15. Bressler NM, Varma R, Doan QV, Gleeson M, Danese M, Bower JK, et al. Underuse of the health care system by persons with diabetes mellitus and diabetic macular edema in the United States. JAMA Ophthalmol. 2014; 132:168–173. PMID: 24357541.

16. Song SJ, Wong TY. Current concepts in diabetic retinopathy. Diabetes Metab J. 2014; 38:416–425. PMID: 25541604.

17. Heier JS, Bressler NM, Avery RL, Bakri SJ, Boyer DS, Brown DM, et al. Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of diabetic macular edema: extrapolation of data to clinical practice. JAMA Ophthalmol. 2016; 134:95–99. PMID: 26512939.
18. Bressler SB, Glassman AR, Almukhtar T, Bressler NM, Ferris FL, Googe JM Jr, et al. Five-year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol. 2016; 164:57–68. PMID: 26802783.

19. Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013; 120:2013–2022. PMID: 23706949.
20. Dubois-Dalcq M, Armstrong R. The cellular and molecular events of central nervous system remyelination. Bioessays. 1990; 12:569–576. PMID: 2080911.

21. Friberg TR, Pandya A, Eller AW. Non-mydriatic panoramic fundus imaging using a non-contact scanning laser-based system. Ophthalmic Surg Lasers Imaging. 2003; 34:488–497. PMID: 14620758.

22. Kaines A, Oliver S, Reddy S, Schwartz SD. Ultrawide angle angiography for the detection and management of diabetic retinopathy. Int Ophthalmol Clin. 2009; 49:53–59. PMID: 19349786.

23. Kan H, Stevens J, Heiss G, Klein R, Rose KM, London SJ. Dietary fiber intake and retinal vascular caliber in the atherosclerosis risk in communities study. Am J Clin Nutr. 2007; 86:1626–1632. PMID: 18065579.

24. Wang L, Wong TY, Sharrett AR, Klein R, Folsom AR, Jerosch-Herold M. Relationship between retinal arteriolar narrowing and myocardial perfusion: multi-ethnic study of atherosclerosis. Hypertension. 2008; 51:119–126. PMID: 17998474.
25. Cheung N, Islam FM, Jacobs DR Jr, Sharrett AR, Klein R, Polak JF, et al. Arterial compliance and retinal vascular caliber in cerebrovascular disease. Ann Neurol. 2007; 62:618–624. PMID: 17918248.

26. Lundberg K, Kawasaki R, Sjolie AK, Wong TY, Grauslund J. Localized changes in retinal vessel caliber after focal/grid laser treatment in patients with diabetic macular edema: a measure of treatment response? Retina. 2013; 33:2089–2095. PMID: 23514802.
27. Nguyen TT, Wang JJ, Sharrett AR, Islam FM, Klein R, Klein BE, et al. Relationship of retinal vascular caliber with diabetes and retinopathy: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2008; 31:544–549. PMID: 18070990.
28. Klein R, Klein BE, Moss SE, Wong TY. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2007; 114:1884–1892. PMID: 17540447.
29. Cheung CY, Ikram MK, Klein R, Wong TY. The clinical implications of recent studies on the structure and function of the retinal microvasculature in diabetes. Diabetologia. 2015; 58:871–885. PMID: 25669631.

30. Mookiah MR, Acharya UR, Fujita H, Tan JH, Chua CK, Bhandary SV, et al. Application of different imaging modalities for diagnosis of diabetic macular edema: a review. Comput Biol Med. 2015; 66:295–315. PMID: 26453760.

31. Sim DA, Keane PA, Tufail A, Egan CA, Aiello LP, Silva PS. Automated retinal image analysis for diabetic retinopathy in telemedicine. Curr Diab Rep. 2015; 15:14. PMID: 25697773.

32. Aghamohamadian-Sharbaf M, Pourreza HR, Banaee T. A novel curvature-based algorithm for automatic grading of retinal blood vessel tortuosity. IEEE J Biomed Health Inform. 2016; 20:586–595. PMID: 25622332.
33. Lee J, Zee BC, Li Q. Detection of neovascularization based on fractal and texture analysis with interaction effects in diabetic retinopathy. PLoS One. 2013; 8:e75699. PMID: 24358105.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download