1. Weetman AP. The immunopathogenesis of chronic autoimmune thyroiditis one century after hashimoto. Eur Thyroid J. 2013; 1:243–250. PMID:
24783026.

2. Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001; 86:930–934. PMID:
11158069.

3. Hansen PS, Brix TH, Iachine I, Kyvik KO, Hegedus L. The relative importance of genetic and environmental effects for the early stages of thyroid autoimmunity: a study of healthy Danish twins. Eur J Endocrinol. 2006; 154:29–38. PMID:
16381988.

4. Brix TH, Hegedus L. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin Endocrinol (Oxf). 2012; 76:457–464. PMID:
22168537.

5. Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y. Immunogenetics of autoimmune thyroid diseases: a comprehensive review. J Autoimmun. 2015; 64:82–90. PMID:
26235382.

6. Villanueva R, Greenberg DA, Davies TF, Tomer Y. Sibling recurrence risk in autoimmune thyroid disease. Thyroid. 2003; 13:761–764. PMID:
14558919.

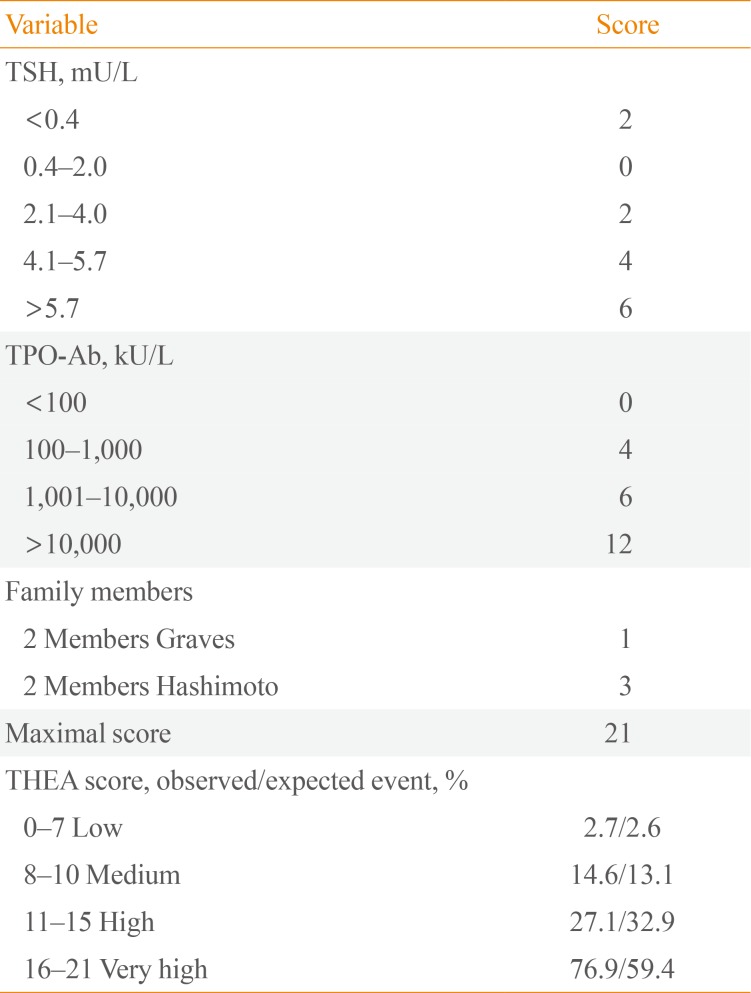
7. Strieder TG, Tijssen JG, Wenzel BE, Endert E, Wiersinga WM. Prediction of progression to overt hypothyroidism or hyperthyroidism in female relatives of patients with autoimmune thyroid disease using the Thyroid Events Amsterdam (THEA) score. Arch Intern Med. 2008; 168:1657–1663. PMID:
18695079.

8. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995; 43:55–68. PMID:
7641412.

9. Flynn RW, MacDonald TM, Morris AD, Jung RT, Leese GP. The thyroid epidemiology, audit, and research study: thyroid dysfunction in the general population. J Clin Endocrinol Metab. 2004; 89:3879–3884. PMID:
15292321.

10. Pedersen IB, Knudsen N, Carle A, Vejbjerg P, Jorgensen T, Perrild H, et al. A cautious iodization programme bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin Endocrinol (Oxf). 2011; 75:120–126. PMID:
21521277.

11. Pedersen IB, Laurberg P, Knudsen N, Jorgensen T, Perrild H, Ovesen L, et al. An increased incidence of overt hypothyroidism after iodine fortification of salt in Denmark: a prospective population study. J Clin Endocrinol Metab. 2007; 92:3122–3127. PMID:
17504896.
12. Aghini Lombardi F, Fiore E, Tonacchera M, Antonangeli L, Rago T, Frigeri M, et al. The effect of voluntary iodine prophylaxis in a small rural community: the Pescopagano survey 15 years later. J Clin Endocrinol Metab. 2013; 98:1031–1039. PMID:
23436921.

13. Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf). 2013; 79:145–151. PMID:
23581474.

14. Strieder TG, Prummel MF, Tijssen JG, Endert E, Wiersinga WM. Risk factors for and prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid disease. Clin Endocrinol (Oxf). 2003; 59:396–401. PMID:
12919165.

15. Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2004; 89:6077–6086. PMID:
15579761.

16. Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Arch Intern Med. 2007; 167:1428–1432. PMID:
17620538.
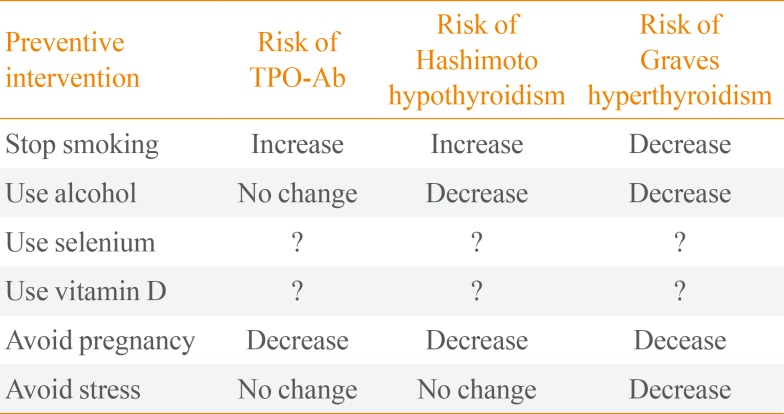
17. Effraimidis G, Tijssen JG, Wiersinga WM. Discontinuation of smoking increases the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies: a prospective study. J Clin Endocrinol Metab. 2009; 94:1324–1328. PMID:
19141579.

18. Carlé A, Bulow Pedersen I, Knudsen N, Perrild H, Ovesen L, Banke Rasmussen L, et al. Smoking cessation is followed by a sharp but transient rise in the incidence of overt autoimmune hypothyroidism: a population-based, case-control study. Clin Endocrinol (Oxf). 2012; 77:764–772. PMID:
22651374.
19. Effraimidis G, Tijssen JG, Wiersinga WM. Alcohol consumption as a risk factor for autoimmune thyroid disease: a prospective study. Eur Thyroid J. 2012; 1:99–104. PMID:
24783003.

20. Carlé A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, et al. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: a population-based case-control study. Eur J Endocrinol. 2012; 167:483–490. PMID:
22802427.

21. Carlé A, Bulow Pedersen I, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, et al. Graves' hyperthyroidism and moderate alcohol consumption: evidence for disease prevention. Clin Endocrinol (Oxf). 2013; 79:111–119. PMID:
23170908.

22. Effraimidis G, Strieder TG, Tijssen JG, Wiersinga WM. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: a prospective study. Eur J Endocrinol. 2011; 164:107–113. PMID:
20956436.

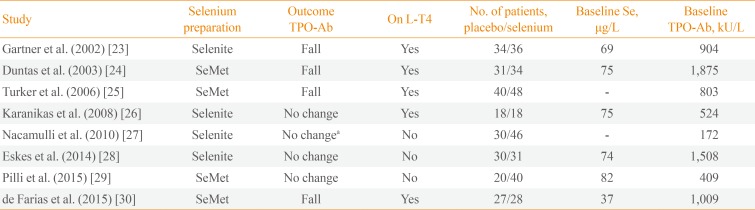
23. Gärtner R, Gasnier BC, Dietrich JW, Krebs B, Angstwurm MW. Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations. J Clin Endocrinol Metab. 2002; 87:1687–1691. PMID:
11932302.
24. Duntas LH, Mantzou E, Koutras DA. Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur J Endocrinol. 2003; 148:389–393. PMID:
12656658.

25. Turker O, Kumanlioglu K, Karapolat I, Dogan I. Selenium treatment in autoimmune thyroiditis: 9-month follow-up with variable doses. J Endocrinol. 2006; 190:151–156. PMID:
16837619.

26. Karanikas G, Schuetz M, Kontur S, Duan H, Kommata S, Schoen R, et al. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid. 2008; 18:7–12. PMID:
18302514.

27. Nacamulli D, Mian C, Petricca D, Lazzarotto F, Barollo S, Pozza D, et al. Influence of physiological dietary selenium supplementation on the natural course of autoimmune thyroiditis. Clin Endocrinol (Oxf). 2010; 73:535–539. PMID:
20039895.

28. Eskes SA, Endert E, Fliers E, Birnie E, Hollenbach B, Schomburg L, et al. Selenite supplementation in euthyroid subjects with thyroid peroxidase antibodies. Clin Endocrinol (Oxf). 2014; 80:444–451. PMID:
23844613.

29. Pilli T, Cantara S, Schomburg L, Cenci V, Cardinale S, Heid EC, et al. IFNγ-inducible chemokines decrease upon selenomethionine supplementation in women with euthyroid autoimmune thyroiditis: comparison between two doses of selenomethionine (80 or 160µg) versus placebo. Eur Thyroid J. 2015; 4:226–233. PMID:
26835425.
30. de Farias CR, Cardoso BR, de Oliveira GM, de Mello Guazzelli IC, Catarino RM, Chammas MC, et al. A randomized-controlled, double-blind study of the impact of selenium supplementation on thyroid autoimmunity and inflammation with focus on the GPx1 genotypes. J Endocrinol Invest. 2015; 38:1065–1074. PMID:
25894865.

31. Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, et al. Selenium and the course of mild Graves' orbitopathy. N Engl J Med. 2011; 364:1920–1931. PMID:
21591944.

32. Negro R, Greco G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab. 2007; 92:1263–1268. PMID:
17284630.

33. Mao J, Pop VJ, Bath SC, Vader HL, Redman CW, Rayman MP. Effect of low-dose selenium on thyroid autoimmunity and thyroid function in UK pregnant women with mild-to-moderate iodine deficiency. Eur J Nutr. 2016; 55:55–61. PMID:
25524327.

34. Rayman MP. Selenium and human health. Lancet. 2012; 379:1256–1268. PMID:
22381456.

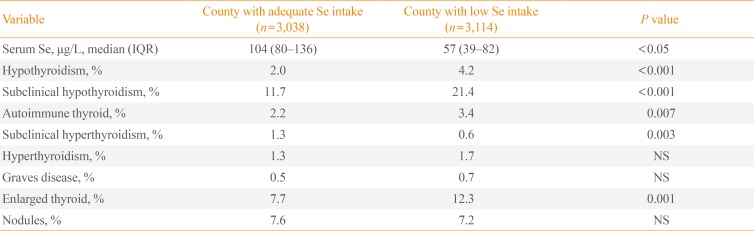
35. Wu Q, Rayman MP, Lv H, Schomburg L, Cui B, Gao C, et al. Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab. 2015; 100:4037–4047. PMID:
26305620.

36. Van Belle TL, Gysemans C, Mathieu C. Vitamin D in autoimmune, infectious and allergic diseases: a vital player? Best Pract Res Clin Endocrinol Metab. 2011; 25:617–632. PMID:
21872803.

37. Wang J, Lv S, Chen G, Gao C, He J, Zhong H, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015; 7:2485–2498. PMID:
25854833.

38. Choi YM, Kim WG, Kim TY, Bae SJ, Kim HK, Jang EK, et al. Low levels of serum vitamin D3 are associated with autoimmune thyroid disease in pre-menopausal women. Thyroid. 2014; 24:655–661. PMID:
24320141.

39. Effraimidis G, Badenhoop K, Tijssen JG, Wiersinga WM. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur J Endocrinol. 2012; 167:43–48. PMID:
22518050.

40. Feng M, Li H, Chen SF, Li WF, Zhang FB. Polymorphisms in the vitamin D receptor gene and risk of autoimmune thyroid diseases: a meta-analysis. Endocrine. 2013; 43:318–326. PMID:
23065592.

41. Tomer Y, Davies TF. Infection, thyroid disease, and autoimmunity. Endocr Rev. 1993; 14:107–120. PMID:
8491150.

42. Wang Z, Zhang Q, Lu J, Jiang F, Zhang H, Gao L, et al. Identification of outer membrane porin f protein of Yersinia enterocolitica recognized by antithyrotopin receptor antibodies in Graves' disease and determination of its epitope using mass spectrometry and bioinformatics tools. J Clin Endocrinol Metab. 2010; 95:4012–4020. PMID:
20484489.
43. Effraimidis G, Tijssen JG, Strieder TG, Wiersinga WM. No causal relationship between Yersinia enterocolitica infection and autoimmune thyroid disease: evidence from a prospective study. Clin Exp Immunol. 2011; 165:38–43. PMID:
21488870.

44. Brix TH, Hansen PS, Hegedus L, Wenzel BE. Too early to dismiss Yersinia enterocolitica infection in the aetiology of Graves' disease: evidence from a twin case-control study. Clin Endocrinol (Oxf). 2008; 69:491–496. PMID:
18284638.
45. Strieder TG, Wenzel BE, Prummel MF, Tijssen JG, Wiersinga WM. Increased prevalence of antibodies to enteropathogenic Yersinia enterocolitica virulence proteins in relatives of patients with autoimmune thyroid disease. Clin Exp Immunol. 2003; 132:278–282. PMID:
12699417.

46. Winsa B, Adami HO, Bergstrom R, Gamstedt A, Dahlberg PA, Adamson U, et al. Stressful life events and Graves' disease. Lancet. 1991; 338:1475–1479. PMID:
1683917.

47. Sonino N, Girelli ME, Boscaro M, Fallo F, Busnardo B, Fava GA. Life events in the pathogenesis of Graves' disease: a controlled study. Acta Endocrinol (Copenh). 1993; 128:293–296. PMID:
8498147.

48. Kung AW. Life events, daily stresses and coping in patients with Graves' disease. Clin Endocrinol (Oxf). 1995; 42:303–308. PMID:
7758236.

49. Radosavljevic VR, Jankovic SM, Marinkovic JM. Stressful life events in the pathogenesis of Graves' disease. Eur J Endocrinol. 1996; 134:699–701. PMID:
8766938.
50. Effraimidis G, Tijssen JG, Brosschot JF, Wiersinga WM. Involvement of stress in the pathogenesis of autoimmune thyroid disease: a prospective study. Psychoneuroendocrinology. 2012; 37:1191–1198. PMID:
22226433.

51. Bülow Pedersen I, Laurberg P, Knudsen N, Jorgensen T, Perrild H, Ovesen L, et al. Lack of association between thyroid autoantibodies and parity in a population study argues against microchimerism as a trigger of thyroid autoimmunity. Eur J Endocrinol. 2006; 154:39–45. PMID:
16381989.

52. Massoudi MS, Meilahn EN, Orchard TJ, Foley TP Jr, Kuller LH, Costantino JP, et al. Prevalence of thyroid antibodies among healthy middle-aged women. Findings from the thyroid study in healthy women. Ann Epidemiol. 1995; 5:229–233. PMID:
7606312.
53. Frank P, Kay CR. Incidence of thyroid disease associated with oral contraceptives. Br Med J. 1978; 2(2):1531. PMID:
728709.

54. Vestergaard P, Rejnmark L, Weeke J, Hoeck HC, Nielsen HK, Rungby J, et al. Smoking as a risk factor for Graves' disease, toxic nodular goiter, and autoimmune hypothyroidism. Thyroid. 2002; 12:69–75. PMID:
11838733.

55. Rotondi M, Pirali B, Lodigiani S, Bray S, Leporati P, Chytiris S, et al. The post partum period and the onset of Graves' disease: an overestimated risk factor. Eur J Endocrinol. 2008; 159:161–165. PMID:
18483014.

56. Stagnaro-Green A, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Negro R. High rate of persistent hypothyroidism in a large-scale prospective study of postpartum thyroiditis in southern Italy. J Clin Endocrinol Metab. 2011; 96:652–657. PMID:
21190974.

57. Effraimidis G, Wiersinga WM. Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur J Endocrinol. 2014; 170:R241–R252. PMID:
24609834.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download