Abstract
Background
Autism spectrum disorder (ASD) encompasses a range of disorders that are characterized by social and communication deficits and repetitive behaviors. This study evaluated the effect of methyl-6-(phenylethynyl)-pyridine (MPEP), an antagonist of the mGluR5 metabotropic glutamate receptor, on memory enhancement in the BTBR T+tf/J (BTBR) mouse strain, which has been recognized as a model of ASD.
Methods
The pharmacological effects of MPEP on memory and motor coordination were assessed using the Morris water maze and rotarod tests in BTBR and C57BL/6J (B6) mice. Furthermore, we performed morphological analyses of cerebellar foliation in BTBR and B6 mice using hematoxylin and eosin staining.
Results
MPEP-treated BTBR mice exhibited improved learning and memory in the Morris water maze test. MPEP administration also improved motor coordination in the rotarod test. However, no significant difference was observed regarding the numbers of Purkinje cells in the cerebella of BTBR versus normal B6 mice.
Conclusion
This study suggests that the mGluR5 antagonist MPEP has the potential to ameliorate learning and memory dysfunction and impaired motor coordination in BTBR mice. These results further suggest that the BTBR mouse model may be useful in pharmacological studies investigating drugs that could potentially alleviate cognitive dysfunction in ASD.
Autism is a neurodevelopmental disorder affecting approximately one in 88 children [1]. The diagnostic symptoms of autism constitute a triad of behavioral deficits consisting of abnormal social interactions, impaired communication, and repetitive and stereotyped patterns of behavior [2]. A strong genetic component of the disorder is evident in the high (70% to 90%) concordance rates for autism in monozygotic twins [3] and the markedly high heritability of individual diagnostic symptoms [4]. However, neuropathologies such as increased gross brain volume [5] and reduced size of the corpus callosum [6] are also frequently associated with this disorder.
In patients with autism spectrum disorder (ASD), impairments in social interaction and communication are usually combined with intellectual disability, increased anxiety and hyperactivity, and abnormal circadian activity, sensory perception, and motor coordination. These phenotypic traits can be investigated using well-established animal models [7]. Furthermore, given that the etiology of autism may involve complex and heterogeneous genetic and experiential interactions, animal models are critical to isolating its underlying mechanisms [8]. Recently, a number of inbred mouse strains have proven themselves particularly useful in relating existing phenotypes to potential genetic or physiological abnormalities [9]. However, the complexity of mouse social behavior presents a challenge to designing tasks to assess social interactions relevant to autism. Such tests quantify social tendencies in mice through a variety of means, such as the duration that a mouse will remain in close proximity to another animal or the frequency of certain stereotypical behaviors. Results from previous social interaction tests have indicated that the inbred BTBR T+tf/J (BTBR) mouse strain displays several social deficits congruent with the diagnostic criteria of autism, including reduced social approach [10], huddling, reduced social investigation, and impaired juvenile play [10]. In comparison to the 'normal' C57BL/6J (B6) mouse strain, BTBR mice also display restricted interest in objects, repetitive grooming [7], and abnormal patterns of scent marking and vocalization [11], which broadly reflect the core symptoms of autism. However, although several studies have reported inconsistent results for anxiety indicators in BTBR mice, the lack of preference for a social stimulus over a non-social stimulus [9,10] and the low levels of social approach displayed by BTBR mice suggest social anxiety in this strain [12]. Because social anxiety is a key symptom among ASD children, we examined the effects of 2-methyl-6-(phenylethynyl)-pyridine (MPEP) in BTBR mice. MPEP is a potent, noncompetitive mGluR5 antagonist that crosses the blood-brain barrier to elicit robust antidepressant-like effects in various rodent models of depression [13], and thus may also have utility in the treatment of ASD.
The aim of this study was to explore the effect of MPEP (10 mg/kg, intraperitoneal) on stereotypical behaviors characteristic to BTBR mice. By examining behavioral and functional outcomes in BTBR versus normal B6 mice, we found that MPEP treatment exerted beneficial effects on spontaneously occurring stereotypical behaviors relevant in the context of a social interaction. Thus, BTBR mice may serve as a useful model to understand the neural mechanisms underlying alterations in behavioral flexibility, as well as to test potential treatments in alleviating ASD symptoms.
Male mice from two inbred strains, B6 and BTBR were purchased from Jackson laboratories (Bar Harbor, ME, USA). They were quarantined and acclimatized for 1 week before use. The mice were housed in a controlled environment (temperature, 22℃±2℃; relative humidity, 55%±5%) with a 12-hour light: dark cycle (lights on at 8:00 AM). Four to five mice were housed together in a polypropylene case. All experiments were performed in accordance with ethical guidelines for the care and use of laboratory animals at the Experimental Animal Center, Hallym University, Korea.
MPEP (Sigma Aldrich, St. Louis, MO, USA) was dissolved in saline (0.9% NaCl). Adult male B6 and BTBR mice weighing 25 to 40 g were administered an intraperitoneal injection of saline vehicle and MPEP (10 mg/kg) 30 minutes before the start of either the Morris water maze or the rotarod test (Fig. 1).
The Morris water maze experiment was conducted as described previously, with some modifications [14]. The Morris water maze is a circular pool (150 cm in diameter and 40 cm in height) with a featureless inner surface. The pool was filled to a depth of 35 cm with water containing non-fat dry milk (22℃±1℃). A transparent platform (6 cm in diameter and 29 cm high) was submerged 1 cm below the surface of opaque water. The tank was placed in a dimly lit, soundproof test room with various visual cues. The first experimental day was dedicated to swimming for 60 seconds in the absence of the platform. During the following 4 days, the mice were given four training sessions per day with the platform in place. During these training trials, mice that located the platform were permitted to remain on it for 10 seconds. If a mouse did not locate the platform within 120 seconds, it was placed on the platform for 10 seconds. After each training session, the animal was returned to its cage and allowed to dry under an infrared lamp. The interval between trials was 15 minutes. During each trial, the time required to find the hidden platform (latency) was recorded using a video camera focused on the full diameter of the pool. Navigation parameters were analyzed using the Ethovision 3.1 video analysis system (Nodulus, Wageningen, Netherlands). One day after the final training trial, mice were individually subjected to a probe trial in which the platform was removed from the pool, and mice were allowed to swim for 120 seconds in search of it. A record was kept of the amount of time spent swimming in the quadrant in which the platform had previously been placed. The following parameters were evaluated during each trial: escape latency (time [second] spent to find the hidden platform), the time (second) spent in the target quadrant (second), the mean distance to platform (cm), and the swim speed (cm/sec). The escape latency, the time spent in the target quadrant, and the distance to platform were used as measures of the development of spatial memory.
Motor coordination was measured using the rotarod test, as described previously [15]. Experimental mice were trained to walk on an accelerating rotarod (diameter, 3 cm; Neuroscience Inc., Tokyo, Japan) with a non-skid surface and the latency to fall was measured for up to 2 minutes. The speed of the rotarod was accelerated from 5 rpm to a maximum speed of 30 rpm. Latency to fall was recorded at the moment the subject fell or stopped walking (clinging to the rod) for three consecutive turns.
The Morris water maze was used to determine the effect of MPEP (10 mg/kg, intraperitoneal) on spatial learning and memory in BTBR and B6 mice. The experimental design is shown in Fig. 1. Escape latency, which was defined as the total time (second) that a mouse spent in the target quadrant before finding the hidden platform, was used as a measure of developing spatial memory. As shown in Fig. 2, MPEP-treated animals exhibited shorter escape latencies as they progressed through training days compared to saline-treated control animals. These data suggest that MPEP (10 mg/kg) administration enhanced the learning and memory of the autism model mice.
The effect of MPEP (10 mg/kg, intraperitoneal) on motor coordination was evaluated using the rotarod test. During the rotarod test, the effects of saline or MPEP treatment on locomotion and the development of sensitized locomotion after repeated treatments were analyzed at 2 and 4 weeks. At 2 weeks, no difference in locomotion was observed within or between the saline- or MPEP-treated groups (Table 1). However, at 4 weeks, MPEP-treated BTBR mice showed a significant improvement in locomotor performance compared to the saline-treated BTBR mice. Although MPEP-treated B6 mice also showed a trend toward improved performance relative to saline-treated B6 mice at 4 weeks, this difference did not reach statistical significance (Table 1). Furthermore, our results show that systemically administered MPEP at a dose of 10 mg/kg increases spontaneous locomotion, which had an effect on the motor coordination function of MPEP-treated BTBR mice.
Routine histological (H&E) staining and microscopic analysis revealed an increase in cerebellar foliation (i.e., larger gross brain volume) in BTBR mice compared to age-matched B6 mice (Fig. 3). However, the number of Purkinje cells in folia 2, 4, and 8 in BTBR versus B6 mice did not differ.
This study examined whether the mGlu5R antagonist MPEP has the potential to improve short-term memory in a mouse model of ASD. Animal models are useful tools in the search for pharmacological treatments for the core symptoms of autism. The mGlu5R antagonist MPEP has been suggested as a potential therapeutic intervention in ASD through its action on the metabotropic glutamate receptor. Although the molecular mechanisms underlying the beneficial effects of mGlu5R antagonists on autism symptoms should be investigated at the molecular pharmacological level, the results of this study strongly suggest that the pharmacological blockade of mGlu5R has neuroprotective effects against striatal excitotoxicity and that MPEP in particular is able to attenuate cognitive dysfunction in autism model mice.
The Morris water-maze learning task is used to assess hippocampus-dependent spatial learning ability [16]. Day-to-day decreases in escape latency reflect learning with respect to reference or long-term memory. Impairment in long-term memory was observed in the MPEP-treated group. The MPEP-treated BTBR mice showed ameliorated memory impairment (i.e., returning the escape latencies to vehicle-treated control group levels). In addition, during the probe trial sessions, MPEP improved both swimming times and distances within the target zone compared to the vehicle-treated control group, indicating spatial memory improvement [17]. Collectively, these behavioral studies suggest that MPEP improves long-term memory in BTBR mice. In the present study, the reduction in accuracy at the longest delay interval observed with a dose higher than that required for anxiolytic activity (i.e., 10 mg/kg) indicates a small effect on working memory. Additionally, at a dose of 10 mg/kg MPEP, we also observed a significant increase in missed trials and escape latencies in BTBR mice (Fig. 2). Furthermore, disruption of the working memory curve also appeared to be delay-independent, although this was not statistically significantly different compared to the saline-treated group. Therefore, 10 mg/kg MPEP did not significantly impair working memory and did not produce a comparable impairment in escape latency in BTBR and B6 mice at 4 weeks (Fig. 2).
The rotarod performance test is used to examine motor coordination in rodents and is often included in test batteries for mouse mutants [18,19]. Consistent with the data from pole- and wire-hanging tests, BTBR mice treated with MPEP remained on the accelerating rod for longer periods compared to MPEP-treated B6 mice at 4 weeks. These findings indicate that MPEP alleviated catalepsy via mGlu5R receptors. In contrast, MPEP (10 mg/kg) did not enhance motor coordination in B6 or BTBR mice at 2 weeks. Moreover, MPEP significantly increased locomotor time in the rotarod test compared to control saline group, whereas MPEP-treated B6 mice showed a non-significant increase in locomotor activity at 4 weeks (Table 1). Thus, MPEP (10 mg/kg) did not cause impairment of motor function or significant alterations in the startle response, but rather improved motor function in BTBR mice during prolonged treatment. Therefore, these differences in sensitivity to MPEP and subsequent effects may be due to differences in its affinity to mGlu5R receptors. The results clearly demonstrate that the marked defect in motor coordination exhibited by BTBR mice was attenuated by MPEP treatment. This defect was apparent only under aggravated conditions (i.e., during the rotarod test), given that BTBR and B6 mice did not display any obvious motor problems such as ataxia, tremor, or difficulty walking at 2 weeks.
At present there is no standard active compound that improves sociability in autism, presenting a major challenge to designing predictive validity into translational testing in mouse models of autism. One of the objectives of this study was to develop a useful experimental design for testing the effects of various compounds on sociability in a mouse model. For this reason, the experimental design presented herein uses two simple behavioral tasks relevant to the first and third diagnostic symptoms of autism, along with a rapid assessment of adverse locomotor side effects. However, we are in the initial stages of applying this strategy to assess the therapeutic potential of treatments for ASD. Therefore, additional studies with a variety of molecular pharmacological approaches are required to assess the mechanisms underlying the enhancement of short-term memory after MPEP treatment in the BTBR mouse model of ASD.
Figures and Tables
 | Fig. 1Experimental design. Treatments were administered by intraperitoneal injection once per day. Group T1 was administered saline vehicle for 4 weeks; group T2 was administered 10 mg methyl-6-(phenylethynyl)-pyridine (MPEP)/kg body weight for 4 weeks. MPEP was re-administered 2 and 4 weeks later, followed by behavioral analysis 30 minutes later. BTBR, BTBR T+tf/J. |
 | Fig. 2Effects of methyl-6-(phenylethynyl)-pyridine (MPEP) on escape latency in the Morris water maze test. Morris water maze training parameters were measured over the course of 5 days at 2 weeks (A) and at 4 weeks (B) after MPEP treatment in both BTBR T+tf/J (BTBR) and C57BL/6J (B6) mice. At 2 weeks, no significant difference in latency time was observed between MPEP-treated and saline-treated B6 mice. When the drug was re-administered 4 weeks after initial administration, the B6 and BTBR group showed no significant difference in latency time. The training trials and probe trials were performed as described in the METHODS. Values are expressed as mean±SEM. aP<0.05; bP<0.01. |
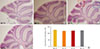 | Fig. 3(A-E) Morphological analyses of cerebellar sections from BTBR T+tf/J (BTBR) and C57BL/6J (B6) mice. The numbers of Purkinje cells were quantified in folia 2, 4, and 8. Photomicrography of cerebellar sections are from C56BL/6J (A, B) and BTBR T+tf/J (C, D). No significant difference was observed in either BTBR or B6 mice (H&E stain, ×20). |
Table 1
Effects of MPEP on Motor Coordination, as Examined via the Rotarod Test, in B6 versus BTBR Mice

| Group | 2 Weeks | 4 Weeks |
|---|---|---|
| B6 saline (n=5) | 22.89±11.99 | 20.06±8.12 |
| B6 MPEP (n=5) | 22.36±2.37 | 31.16±3.19 |
| BTBR saline (n=7) | 14.40±15.30 | 14.80±10.29 |
| BTBR MPEP (n=7) | 19.09±22.38 | 44.54±18.55a |
Values are expressed as mean±SEM. At the 2- and 4-week trials, no difference was observed between MPEP-treated B6 mice versus the saline-treated B6 controls. However, among BTBR mice, MPEP treatment resulted in a significant increase in motor performance during the 4-week trial, compared to saline-treated BTBR controls. The rotating speed was 5 rpm. MPEP, methyl-6-(phenylethynyl)-pyridine; B6, C57BL/6J; BTBR, BTBR T+tf/J.
aP<0.05.
ACKNOWLEDGMENTS
This work was supported by Hallym University Research Fund (HRF-201404-010) and by the Priority Research Centers Program (NRF-2009-0094071) funded by the Ministry of Education, Science, and Technology.
References
1. Manning-Courtney P, Murray D, Currans K, Johnson H, Bing N, Kroeger-Geoppinger K, Sorensen R, Bass J, Reinhold J, Johnson A, Messerschmidt T. Autism spectrum disorders. Curr Probl Pediatr Adolesc Health Care. 2013; 43:2–11.
2. Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000; 28:355–363.
3. Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001; 2:943–955.
4. Ronald A, Happe F, Bolton P, Butcher LM, Price TS, Wheelwright S, Baron-Cohen S, Plomin R. Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry. 2006; 45:691–699.
5. Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. Am J Psychiatry. 1995; 152:1145–1149.
6. Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Arch Neurol. 1995; 52:794–801.
7. Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010; 35:976–989.
8. Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008; 13:4–26.
9. Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007; 176:4–20.
10. Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007; 1:1.
11. Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008; 3:e3067.
12. Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007; 176:21–26.
13. Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol. 2007; 17:172–179.
14. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47–60.
15. Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996; 86:159–171.
16. Barnes CA, Danysz W, Parsons CG. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. Eur J Neurosci. 1996; 8:565–571.
17. Blokland A, Geraerts E, Been M. A detailed analysis of rats' spatial memory in a probe trial of a Morris task. Behav Brain Res. 2004; 154:71–75.
18. Tarantino LM, Gould TJ, Druhan JP, Bucan M. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mamm Genome. 2000; 11:555–564.
19. Brandon EP, Logue SF, Adams MR, Qi M, Sullivan SP, Matsumoto AM, Dorsa DM, Wehner JM, McKnight GS, Idzerda RL. Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice. J Neurosci. 1998; 18:3639–3649.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download