Abstract
A 31-year-old woman was referred to our hospital with symptoms of hypertension and bilateral adrenocortical masses with no feature of Cushing syndrome. The serum aldosterone/renin ratio was elevated and the saline loading test showed no suppression of the plasma aldosterone level, consistent with a diagnosis of primary hyperaldosteronism. Overnight and low-dose dexamethasone suppression tests showed no suppression of serum cortisol, indicating a secondary diagnosis of subclinical Cushing syndrome. Adrenal vein sampling during the low-dose dexamethasone suppression test demonstrated excess secretion of cortisol from the left adrenal mass. A partial right adrenalectomy was performed, resulting in normalization of blood pressure, hypokalemia, and high aldosterone level, implying that the right adrenal mass was the main cause of the hyperaldosteronism. A total adrenalectomy for the left adrenal mass was later performed, resulting in a normalization of cortisol level. The final diagnosis was bilateral adrenocortical adenomas, which were secreting aldosterone and cortisol independently. This case is the first report of a concurrent cortisol-producing left adrenal adenoma and an aldosterone-producing right adrenal adenoma in Korea, as demonstrated by adrenal vein sampling and sequential removal of adrenal masses.
Primary hyperaldosteronism is the most common cause of secondary hypertension, characterized by excessive production of aldosterone relatively independent of the renin-angiotensin system. Patients with hyperaldosteronism typically present with one or more symptoms, including hypokalemia, muscle weakness and cramping, headaches, palpitations, polydipsia, polyuria, and nocturia [1]. Adrenal vein sampling is recommended for most patients requesting surgical treatment as a means of differentiating between unilateral and bilateral forms of primary hyperaldosteronism [2].
The term "subclinical Cushing syndrome" is used to describe autonomous cortisol secretion in patients who otherwise lack the typical signs and symptoms of hypercortisolism (Cushing syndrome). Although difficult to detect clinically, 5% to 20% of all adrenocortical adenomas are responsible for the cortisol overproduction [3]. Although the likelihood of subclinical Cushing syndrome progressing to overt Cushing syndrome remains a topic of considerable debate, subclinical Cushing syndrome alone represents an important clinical condition, as it is strongly associated with several cardiovascular risk factors, including obesity, arterial hypertension, type 2 diabetes mellitus, dyslipidemia, and osteoporosis [45].
Recent reports have suggested an increase in the frequency of synchronous excretion of aldosterone and cortisol from adrenal masses. Although most of these reports describe solitary or multiple adrenocortical adenomas secreting both aldosterone and cortisol simultaneously, cases of independent secretion of each hormone from separate adenomas are extremely rare.
Herein, we report an extremely rare case of a patient presenting with primary hyperaldosteronism and subclinical Cushing syndrome caused by bilateral adrenocortical adenomas, each secreting different types of hormones (cortisol from the left adenoma and aldosterone from the right).
A 31-year-old woman was diagnosed with hypertension by a private clinic in August 2013, based upon a systolic blood pressure of 160 mm Hg and she was prescribed antihypertensive drugs to treat the condition. Initial therapy consisted of hydrochlorothiazide 12.5 mg and losartan 50 mg daily. After 1 week, her blood pressure was still high, so the doctor changed her medication to amlodipine 5 mg, losartan 100 mg, and indapamide 2.5 mg per day. Five days after initiating this second regimen of drugs, she developed pretibial pitting edema, and thorough workup was done for the symptom. There were bilateral adrenal masses on abdominal-pelvic computed tomography (CT). Routine blood tests revealed the following: potassium 2.2 mmol/L, plasma renin activity (PRA) 0.12 ng/mL/hr, plasma aldosterone concentration (PAC) 16.76 ng/dL, and an aldosterone/renin ratio (PAC/PRA) >30, indicative of primary hyperaldosteronism.
Following her possible diagnosis of primary hyperaldosteronism, the patient was referred to our hospital for additional testing. At the time of admission, her blood pressure was 155/111 mm Hg, height was 164.5 cm, and weight was 63.5 kg (body mass index, 23.47 kg/m2). Pretibial pitting edemas were detected, but no physical signs of Cushing syndrome were observed.
A routine blood test at the time of admission showed the following: white blood cell 6,900/µL, hemoglobin 13.5 g/dL, platelet 351,000/µL, total protein 6.9 g/dL, albumin 4.1 g/dL, aspartate aminotransferase 76 U/L, alanine aminotransferase 126 U/L, blood urea nitrogen 7.3 mg/dL, creatinine 0.71 mg/dL, sodium 141 mmol/L, potassium 2.9 mmol/L, chloride 101 mmol/L, total cholesterol 210 mg/dL, triglyceride 104 mg/dL, high density lipoprotein 50 mg/dL, low density lipoprotein 141 mg/dL, and hemoglobin A1c 5.9%. Biochemical evaluation of pheochromocytoma showed no excessive catecholamine production. The transtubular potassium concentration gradient was 12.64%, suggesting the possibility of hyperaldosteronism or hypercortisolism. PAC and PRA were 24.4 ng/dL and 0.76 ng/mL/hr, respectively, and the PAC/PRA ratio was 32.11 (Table 1). After correcting for hypokalemia, a saline loading test was performed, which revealed no suppression of the aldosterone level after saline administration, consistent with a diagnosis of hyperaldosteronism (the basal PAC 24.4 ng/dL increased to 27.3 ng/dL after a 2 L saline infusion). Dexamethasone suppression tests (DSTs) resulted in no suppression of cortisol levels (Table 2). Adrenal CT demonstrated two bilateral adrenal masses in each of the adrenal glands, including a 1.6-cm mass on the right and a 2-cm mass on the left adrenal gland (Fig. 1A, B). An adrenal cortical scan using iodine-131-6β-iodomethyl-19-norcholesterol (NP-59) showed focally increased radioactive uptakes in the bilateral adrenal gland areas, with a substantially higher uptake on the left side (Fig. 1C).
Bilateral adrenal venous sampling (AVS) with adrenocorticotropic hormone (ACTH) stimulation (Table 3) revealed inadequate right adrenal venous blood sampling (right adrenal vein cortisol level/peripheral blood cortisol level [CRt/CP] <10), whereas the cortisol concentration in the left adrenal venous blood showed successful sampling on the left side. One explanation for this difference may be that the low level of CRt was the result of suppressed cortisol secretion due to excessive cortisol secretion from the left adrenal mass. The left adrenal vein aldosterone/cortisol (ALt/CLt) ratio was lower than that of peripheral blood, implying the possibility of suppressed aldosterone secretion from this side caused by a contralateral adrenal mass which might secrete excessive aldosterone [6]. Four days after the first AVS, a second AVS was performed using low-dose DST but without ACTH infusion (Table 4). This time, we also measured the epinephrine levels in the both adrenal veins and peripheral blood; a large peripheral-to-adrenal gradient of epinephrine assured successful catheterization on both sides [78]. CLt/CP testing confirmed that the left adrenal mass was a cortisol-secreting adenoma [910].
Even though those two AVS results appeared to provide clear evidence of the lateralization of functioning adrenal masses, such an independent functioning of bilateral masses was highly suspicious. We therefore concluded that sequential adrenalectomies, rather than concurrent operations, would be beneficial for making a definitive diagnosis. Moreover, the patient agreed to do sequential operations. The first procedure was a laparoscopic partial adrenalectomy of the right adrenal gland to remove the putative aldosterone-producing mass, with the goal of controlling hypertension and hypokalemia. A firm, 1.5-cm ovoid, well-circumscribed, bright yellow tumor mass lacking necrosis or hemorrhage was arising from the cortex of the adrenal gland in the pathologic specimen. Within 24 hours after surgery, the patient's blood pressure had fallen to 110/80 mm Hg without antihypertensive medication, serum potassium normalized at 4.5 mmol/L, and PAC decreased to 3 ng/dL. These large postoperative changes strongly suggested that the right adrenal adenoma was producing excessive aldosterone. By day 3, an overnight DST continued to show no cortisol suppression, indicating persistent secretion of corticosteroid from the left adrenal mass. To limit the likelihood of subclinical Cushing syndrome progressing to overt Cushing syndrome, we opted to completely remove the left adrenal gland as a means of controlling hypercortisolism. The excised portion of the adrenal mass contained an encapsulated brown solid mass.
The patient began taking prednisolone 10 mg once a day for steroid replacement 1 day after the second surgery. By 2 months she had lost 5 kg of body weight. At that time, a rapid ACTH test revealed adrenal insufficiency (Table 5). At 16 months after surgery, the patient has gradually reduced her prednisolone intake to 5 mg every other day. Her blood pressure and serum potassium levels have remained within normal ranges, without the need for additional antihypertensive medications or potassium supplements.
Primary hyperaldosteronism is one of the main causes of secondary hypertension, characterized by an excessive production of aldosterone independent of the renin-angiotensin system and a lack of response to the sodium loading test. The prevalence of primary hyperaldosteronism in nonselected hypertensive patients and adrenal incidentaloma is 10% and 1.8%, respectively [211]. The vast majority of patients with hyperaldosteronism exhibit either a unilateral aldosterone-producing adenoma or idiopathic bilateral adrenal hyperplasia [1]; in comparison, bilateral aldosterone-producing adenomas are rarely reported [12].
Relatively young patients presenting with functioning adrenocortical adenomas, particularly those producing aldosterone, are treated with surgical excision as a means of achieving symptom control, including hypertension and persistent hypokalemia. Thus, recognizing the lateralization of aldosterone secretion in cases of bilateral adenomas is important. In these cases, AVS is considered the gold standard for detecting aldosterone production in bilateral adrenal hyperplasia and for distinguishing the lateralization of aldosterone secretion [8].
Evidence of subclinical Cushing syndrome in the presence of primary hyperaldosteronism has been reported in several studies [1314], most of which described a solitary adrenocortical adenoma secreting both aldosterone and cortisol simultaneously. Oki et al. [15] reported a case of bilateral adrenocortical adenomas in which the left adenoma secreted cortisol or both cortisol and aldosterone, while the right adenoma secreted both aldosterone and cortisol, as confirmed by AVS and pathological studies using immunohistochemical stains. As primary hyperaldosteronism has been shown to coexist with hypercortisolism, patients should also be tested for hypercortisolism in cases of suspected primary hyperaldosteronism.
However, cases of multiple simultaneous adrenocortical adenomas secreting each hormone independently are extremely rare, particularly when those adenomas are located in different adrenal glands. As far as we can find from the database of PubMed and KoreaMed, only four cases have been reported in English, Japanese, and Korean [16171819]. The first such case in Korea was reported by Choi et al. [16], who described a patient having primary hyperaldosteronism and Cushing syndrome with bilateral adrenal masses. AVS testing was unsuccessful in this patient, leading to simultaneous removal of the bilateral adrenal masses. As the removal of these masses was performed together, the identities of hormones secreted from each mass were unclear. In contrast, our sequential surgical approach was able to identify discrete secretory functions for each mass. This case is therefore the first report in Korea describing bilateral adrenocortical adenomas functioning independently that resulted in subclinical Cushing syndrome and primary hyperaldosteronism. A similar report by Onoda et al. [19] described a case of synchronous Cushing syndrome and primary hyperaldosteronism with multiple adrenocortical adenomas secreting each hormone individually. AVS showed excess secretion of cortisol and aldosterone from the right and left tumors, respectively. A bilateral laparoscopic partial adrenalectomy was performed, followed by in situ hybridization for mRNAs in each pathologic specimen so as to demonstrate independent production of cortisol (both HSD3B2 and CYP17A1 mRNAs expression) and aldosterone (both HSD3B2 and CYP11B mRNAs expression).
To our knowledge, this is the first case in Korea describing the co-presence of a corticosteroid-producing left adrenal adenoma and an aldosterone-producing right adrenal adenoma, which was demonstrated biochemically and clinically by AVS and the sequential removal of adrenal masses.
Based upon the findings described here, AVS may be useful for accurately diagnosing patients with hypercortisolism accompanied by bilateral adrenal masses [910]. In the second round of AVS testing using low-dose DST and without ACTH stimulation, an elevated CLt/CP ratio revealed autonomous cortisol production by the left adrenocortical adenoma. A combination of a basal hormone test in conjunction with AVS results confirmed the coexistence of excessive cortisol and aldosterone secretion.
While a few reports have demonstrated adenomas producing cortisol and aldosterone using CYP11B1/B2 immunohistochemical staining, our analyses were limited to standard clinical and biochemical tests due to a lack of the availability of immunohistochemical tests in Korea. Nevertheless, surgical findings (Fig. 2) were compatible with features of both aldosteronomas (often a small and solitary mass, a transverse section demonstrating a lipid-rich, bright yellow color) and adenomas causing Cushing syndrome (well defined with apparent encapsulation, the cut surface appearing as yellow or irregular mottled areas or showing diffuse pigmentation) [20].
In conclusion, we have described a unique case of bilateral adrenocortical adenomas. Diagnoses were confirmed by AVS and postoperative changes in clinical features and laboratory tests, which demonstrated the co-presence of a cortisol-producing left adrenal adenoma and an aldosterone-producing right adrenal adenoma.
Such cases of bilateral adrenocortical adenomas with independent production of different hormones are extremely rare, and in this regard, this case report is the first important finding of this disease in Korean patients.
Figures and Tables
Fig. 1
Adrenal Images. (A, B) Adrenal computed tomography. Axial images showed bilateral adrenal masses (arrows), including a 1.6-cm mass on the right adrenal gland (A) and a 2-cm mass on the left (B). (C) Adrenal NP-59 scan demonstrated focally increased radioactive uptakes in the bilateral adrenal gland areas (arrow, arrowhead), especially on the left side (arrowhead).
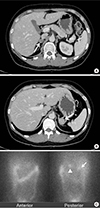
Fig. 2
Adrenalectomy specimens (gross and microscopic findings). (A) The right adrenal mass was a 1.4×1.4×1.2-cm ovoid, bright yellow, and well-circumscribed mass arising from the cortex. (B) Microscopically, the right adrenal mass consisted primarily of clear cells (H&E stain, ×200). (C) The left adrenal mass was brown, solid in appearance, and encapsulated. (D) The left adrenal mass was composed of both clear cells and eosinophilic compact cells (H&E stain, ×200).
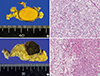
Table 1
Laboratory Data on Admission
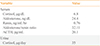
| Variable | Value |
|---|---|
| Serum | |
| Cortisol, µg/dL | 6.8 |
| Aldosterone, ng/dL | 24.4 |
| Renin, ng/mL/hr | 0.76 |
| Aldosterone/renin ratio | 32.1 |
| ACTH, pg/mL | 20.1 |
| Urine | |
| Cortisol, µg/day | 35 |
Table 2
Results of the Dexamethasone Suppression Test

| Variable | Value |
|---|---|
| 1 mg DST | |
| Serum cortisol, µg/dL | 8 |
| Low-dose DST | |
| Urine cortisol, µg/day | 96 |
| Serum cortisol, µg/dL | 11.1 |
Table 3
First Adrenal Vein Sampling with Adrenocorticotropic Hormone Stimulation

| Aldosterone, ng/dL | Cortisol, µg/dL | A/C ratio at each point | |
|---|---|---|---|
| Sampling point | |||
| Peripheral vein | 59 | 12 | 4.92 |
| Right adrenal vein | 358.4 | 10.9 | Catheterization failure |
| Left adrenal vein | 366.3 | 323 | 1.13 |
Table 4
Second Adrenal Vein Sampling without Adrenocorticotropic Hormone Stimulation, with Low Dose Dexamethasone Suppression Test

References
1. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007; 66:607–618.
2. Kim SW. Primary aldosteronism. Korean J Med. 2012; 82:396–402.
3. De Leo M, Cozzolino A, Colao A, Pivonello R. Subclinical Cushing's syndrome. Best Pract Res Clin Endocrinol Metab. 2012; 26:497–505.
4. Terzolo M, Pia A, Reimondo G. Subclinical Cushing's syndrome: definition and management. Clin Endocrinol (Oxf). 2012; 76:12–18.
5. Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, et al. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014; 99:827–834.
6. Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009; 151:329–337.
7. Levinson PD, Zadik Z, Hamilton BP, Mersey JH, White RI, Kowarski AA. Adrenal vein epinephrine levels: a useful aid in venous sampling for primary aldosteronism. Ann Intern Med. 1982; 97:690–693.
8. Lee JS, Kang MY, Kim SW, Shin CS, Kim SY, Chung JW. The clinical implication and problems of adrenal vein sampling in patients with primary aldosteronism. J Korean Endocr Soc. 2007; 22:428–435.
9. Young WF Jr, du Plessis H, Thompson GB, Grant CS, Farley DR, Richards ML, et al. The clinical conundrum of corticotropin-independent autonomous cortisol secretion in patients with bilateral adrenal masses. World J Surg. 2008; 32:856–862.
10. Ku EJ, Hong AR, Kim YA, Bae JH, Chang MS, Kim SW. Adrenocorticotropic hormone-independent cushing syndrome with bilateral cortisol-secreting adenomas. Endocrinol Metab (Seoul). 2013; 28:133–137.
11. Cho YY, Suh S, Joung JY, Jeong H, Je D, Yoo H, et al. Clinical characteristics and follow-up of Korean patients with adrenal incidentalomas. Korean J Intern Med. 2013; 28:557–564.
12. Wu VC, Chueh SC, Chang HW, Lin WC, Liu KL, Li HY, et al. Bilateral aldosterone-producing adenomas: differentiation from bilateral adrenal hyperplasia. QJM. 2008; 101:13–22.
13. Fujimoto K, Honjo S, Tatsuoka H, Hamamoto Y, Kawasaki Y, Matsuoka A, et al. Primary aldosteronism associated with subclinical Cushing syndrome. J Endocrinol Invest. 2013; 36:564–567.
14. Hiraishi K, Yoshimoto T, Tsuchiya K, Minami I, Doi M, Izumiyama H, et al. Clinicopathological features of primary aldosteronism associated with subclinical Cushing's syndrome. Endocr J. 2011; 58:543–551.
15. Oki K, Yamane K, Sakashita Y, Kamei N, Watanabe H, Toyota N, et al. Primary aldosteronism and hypercortisolism due to bilateral functioning adrenocortical adenomas. Clin Exp Nephrol. 2008; 12:382–387.
16. Choi SE, Kim YC, Noh DY, Youn YK, Oh SK. A case of bilateral adrenal cortical adenomas causing Cushing's syndrome and primary aldosteronism. J Korean Surg Soc. 1998; 55:769–774.
17. Nagae A, Murakami E, Hiwada K, Kubota O, Takada Y, Ohmori T. Primary aldosteronism with cortisol overproduction from bilateral multiple adrenal adenomas. Jpn J Med. 1991; 30:26–31.
18. Okura T, Miyoshi K, Watanabe S, Kurata M, Irita J, Manabe S, et al. Coexistence of three distinct adrenal tumors in the same adrenal gland in a patient with primary aldosteronism and preclinical Cushing's syndrome. Clin Exp Nephrol. 2006; 10:127–130.
19. Onoda N, Ishikawa T, Nishio K, Tahara H, Inaba M, Wakasa K, et al. Cushing's syndrome by left adrenocortical adenoma synchronously associated with primary aldosteronism by right adrenocortical adenoma: report of a case. Endocr J. 2009; 56:495–502.
20. Lack EE, Wieneke J. Chapter 19, Tumors of the adrenal gland. Diagnostic histopathology of tumors. 4th ed. Philadelphia: Elsevier Health Sciences;2013. p. 1294–1325.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download