Abstract
Vertebral fractures and trabecular bone loss have dominated thinking and research into the pathogenesis and the structural basis of bone fragility during the last 70 years. However, 80% of all fractures are non-vertebral and occur at regions assembled using large amounts of cortical bone; only 20% of fractures are vertebral. Moreover, ~80% of the skeleton is cortical and ~70% of all bone loss is cortical even though trabecular bone is lost more rapidly than cortical bone. Bone is lost because remodelling becomes unbalanced after midlife. Most cortical bone loss occurs by intracortical, not endocortical remodelling. Each remodelling event removes more bone than deposited enlarging existing canals which eventually coalesce eroding and thinning the cortex from 'within.' Thus, there is a need to study the decay of cortical as well as trabecular bone, and to develop drugs that restore the strength of both types of bone. It is now possible to accurately quantify cortical porosity and trabecular decay in vivo. The challenges still to be met are to determine whether measurement of porosity identifies persons at risk for fracture, whether this approach is compliments information obtained using bone densitometry, and whether changes in cortical porosity and other microstructural traits have the sensitivity to serve as surrogates of treatment success or failure.
One of the great pioneers of bone biology, Fuller Albright, reported the common occurrence of vertebral fractures in postmenopausal women [1]. The only method of quantifying loss of bone at the time was radiography. Translucency of the vertebral body suggested that bone fragility was the result of trabecular bone loss because the volume of the vertebral body is largely trabecular in configuration; there is only a thin rim of cortical bone forming the shell of this structure [23].
The notion of trabecular bone as the main source of bone loss, and by inference, the main cause of bone fragility, was reinforced by findings using histomorphometry of iliac crest bone biopsy specimens which showed deficits in trabecular bone volume fraction [456]. Likewise, measurement of the spine using dual photon absorptiometry showed better separation of women with and without vertebral fractures than measurement of appendicular cortical bone using single photon absorptiometry [78910].
This 'trabeculo-centric' view of bone fragility was further supported by the results of prospective studies suggesting that trabecular bone is remodelled and lost more rapidly than cortical bone. Trabecular bone loss is usually more rapid, in part, because the spatial configuration confers a larger surface area/bone matrix volume than found with cortical bone [111213].
Remodelling may be signaled within mineralized matrix, marrow, the circulation or centrally, but initiation of remodelling requires a surface. The large surface area facilitates initiation of remodelling. The trabecular bone matrix volume is small and is rapidly lost as the many remodelling events perforate the plates and irreversibly destroy the network of plates and sheets of this cancellous structure (Fig. 1).
The above data and other studies positioned trabecular bone loss as an important determinant of bone fragility but diverted attention away from cortical bone. However, during the last decade attention has been redirected to now better recognize that cortical bone destruction is a determinant of whole bone strength. Moreover, attention has been directed specifically to cortical porosity as this measureable microstructural feature is a permanent record of bone loss and a predictor of bone fragility. While methods of quantifying cortical porosity are available, several challenges remain and some of these are discussed below.
Just as bone mass late in life is determined by the net amount gained during growth and the net amount lost during advancing age, porosity late in life is also determined by the net porosity established during growth and any net increase in porosity excavated during advancing age. As a long bone increases in length by endochondral apposition and width of the diaphysis by periosteal apposition during growth, mass is minimized by the resorptive activity upon the endocortical surface which removes some of the bone deposited by periosteal apposition. This resorptive activity enlarges the medullary canal and shifts the cortical matrix volume radially, a shift that achieves a given resistance to bending with less material because bending strength is a fourth-power function of the radial distance of a unit volume of bone from its neutral axis; less mass achieves more strength when it is deposited further from the neutral axis [14]. Concurrently, intracortical remodelling forms secondary osteons, each with their Haversian and Volkmann canals which transmit vessels and nerves but also serve to minimize mass [151617]. However, increase in porosity in cortex positioned distant from the neutral axis confers greater loss of bending strength than the same loss of material from more centrally positioned bone matrix.
The size and number of osteons and their Haversian and Volkmann canals assembled during secondary osteonal bone formation form the fluid filled void volume within the cortical compartment (defined externally by the periosteal envelope and internally by the endocortical envelope). The term cortical 'pore' or 'porosity' is a somewhat misleading, porosity of cortical bone is not like a cave or hole in Swiss cheese. In a cross section of cortical bone, the porosity is formed by canals seen as 'pores' or 'porosity' in that cross section. The canals are lined by the intracortical component of bone's inner or endosteal surface. This lining is the location upon which intracortical remodelling takes place during growth and advancing age and as remodelling becomes unbalanced during aging, this is the location of most cortical bone loss.
The ends or the metaphyseal regions are formed very differently to the diaphysis of long bones during growth. The cortex is trabecular in origin. Formation of the cortex occurs by 'corticalization' of trabeculae. As trabeculae emerge from the growth plate, adjacent trabeculae in the periphery of the growth plate coalesce; they fuse and contribute, with the periosteal collar, to form cortical bone while the centrally placed trabeculae form the metaphyseal trabecular compartment [181920212223]. The transition between corticalising (coalescing) trabeculae laterally and trabeculae in the medullary canal is indistinct; it is gradual and forms a 'transitional or corticotrabecular zone' between the compact-appearing cortex radially and the medullary canal and trabecular bone centrally (Fig. 2) [2425].
Fragility fractures commonly involve regions containing both cortical and trabecular bone like the metaphyses of the distal radius, proximal humerus, distal tibia, and proximal femur [26]. One third of children have fractures and ~50% of the fractures involve the distal forearm [27]. Forearm fractures are also the most common fractures in pre- and postmenopausal women [28]. The use of minimal mass to assemble this microstructure may come at a price of a low safety margin.
Fragility may result during growth if fewer trabeculae are generated from the growth plate, if they are thinner or fail to coalescence to form the cortex but rather fuse incompletely causing cortical porosity due to failed coalescence. For example, in 110 girls aged 7 to 18 years, imaging of the distal radius using high resolution peripheral computed tomography (HR-pQCT), suggested that that in controls, fewer or thinner trabeculae were associated with a smaller and more porous cortical area. Girls with forearm fractures had 0.3 to 0.7 standard deviations (SD) fewer or thinner trabeculae and higher porosity than controls; a one SD trait difference conferred odds ratio (OR; 95% confidence interval [CI]) for fracture ranging from 1.56 (95% CI, 1.01 to 2.44) to 2.5 (95% CI, 1.62 to 4.58) [22].
Studies of bone microarchitecture in baboons demonstrate that ~60% of the variation in cortical microstructure is accounted for by genetic factors [2930]. Identical twins have higher correlations in cortical porosity than non-identical twins and higher correlations between cortical porosity in one twin versus medullary area in the co-twin than non-identical twins [3132]. These studies, and associations between trabecular morphology in daughters and cortical morphology in mothers, suggest that differences in trabecular and cortical morphology between individuals are largely the result of shared genetic factors or shared environmental factors.
Around midlife in women, bone remodeling becomes unbalanced and rapid. The negative bone balance is produced by deposition of less bone than was resorbed by each remodelling event [3334]. This imbalance leaves a small bone matrix volume deficit focally producing focal structural deterioration; trabeculae thin, perforate and eventually disappear completely. Eventually, the bone loss from the trabecular compartment stops because few trabeculae are left to lose [35]. Bone loss occurs more rapidly from the trabecular compartment, but as only 20% of total bone matrix volume is trabecular and 80% of the skeleton is cortical, in absolute terms, 70% of all appendicular bone loss arises from the cortical compartment even though the cortical bone loss usually proceeds more slowly than trabecular bone loss [1125].
The mineralized cortical bone matrix is enveloped by the periosteal envelope externally, the intracortical surface of canals traversing it and the endocortical surface adjacent to the medullary canal (Fig. 3). Remodelling occurs upon the intracortical and endocortical surfaces [3637]. Cortical bone is lost more slowly than trabecular bone, at least initially, because it is less accessible to being remodelled. It has a high matrix volume enveloped with a smaller surface area so there is less surface area per unit matrix volume available to initiate matrix remodeling.
Each time a remodelling event is initiated upon a canal surface, refilling of that cavity is incomplete leaving the canal cross-section slightly wider at that point. With chronicity, the canals enlarge, the surface area enlarges and so more area is available for remodelling to be initiated upon. Remodelling rate increases in cortical bone as more and more porosity provides more surface area for remodeling to be initiated upon. Remodelling becomes self-perpetuating, more remodelling occurs of an ever-decreasing cortical matrix volume so the rate of cortical bone loss accelerates.
Cortical bone is also eroded by unbalanced and rapid remodelling upon the endocortical surface but most cortical bone loss is the result of intracortical remodelling initiated upon the canal surfaces [25]. Loss of bone from the surfaces of canals traversing cortex adjacent to the medullary canal results in the canals coming closer together and they eventually coalesce producing larger irregular pores in cross section, a morphological change that can now be quantified in vivo, but not without some challenges [253839].
Porosity increases throughout the cortex but cavitation of the inner cortex adjacent to the medullary canal 'trabecularises' this cortex (the opposite of corticalisation of trabeculae during growth). Trabecularisation forms the 'transitional or cortico-trabecular junctional' zone which has a surface/matrix volume ratio that is intermediate between that of cortical and trabecular bone. By convention, cortical bone comprises ~70% mineralized matrix, and ~30% void volume formed mainly by the canals traversing it. Trabecular bone comprised of 10% to 30% mineralized bone matrix fashioned as plates and rods occupying the medullary canal which constitutes 70% to 90% void volume.
The relevance of cortical porosity is in its effects on bone strength. Cortical bone volume is positioned radially, distant from the neutral axis, and more so in taller individuals. Resistance to bending increases to the fourth power of its radius [40]. Even in the vertebral body, 30% to 60% of the mass is displaced peripherally as a thin cortical shell and 45% to 75% of the axial load in compression is carried by the cortex [41]. In the femoral neck (FN), removing the trabeculae decreased fracture load by only 10% [42]. The cortex carries most of the load but this fraction of load carried is ~90% in the distal FN and ~30% in the proximal part [4344]. With loss of bone, the proportion of the total load carried by the cortex increases as trabeculae are lost and this load is less well tolerated as cortical bone also deteriorates [45].
As apparent density (the reciprocal or porosity) decreases in cortical and trabecular bone, stiffness decreases as a 7th power function in cortical bone but only to the 3rd power in trabecular bone [46]. Even a small change in porosity compromises stiffness to a greater extent than a similar increase in a porous structure like trabecular bone. A 4% rise in porosity increases crack propagation by 84% [47]. An increase in porosity from 4% to 10% more than halves the peak stress tolerated by bone [48]. Bone's ability to deform without cracking decreases 3-fold as porosity increases from 4% to 20%. In femoral cortical bone samples, changes in intracortical porosity explained 70% to 80% of the variation in stiffness assessed using scanning acoustic microscopy [4950515253].
The profound loss of strength resulting from cortical bone loss contributes to the burden of fractures. In a population-based study of 100 postmenopausal women aged >50 years with a distal forearm fracture matched with 105 controls, women with forearm fractures had increased cortical porosity and decreased trabecular bone volume fraction. Both predicted forearm fractures, but only cortical porosity did so independently of the ultra distal radius or FN bone mineral density (BMD). The diagnostic threshold for osteoporosis (T-score<-2.5 SD) captured high cortical porosity and low trabecular bone volume fraction whether a forearm fracture was present or not. Thus, after finding a BMD T-score <-2.5 at the ultra distal radius, a measurement of porosity did not identify more women with forearm fractures than measuring areal BMD alone [54].
Most fractures in the community arise from the larger segment of the population with a BMD T-score less severely reduced than <-2.5 SD [555657]. In this study [54], at the ultra distal radius, 62% of women with forearm fractures did not have osteoporosis (38% had osteopenia, 24% had normal BMD). At the FN, 91% did not have osteoporosis (72% had osteopenia and 19% had normal BMD). As a group, women with osteopenia (those with and without forearm fractures) were not at increased risk for fracture; neither ultra distal radius nor FN osteopenia alone were associated with fracture, measuring microstructure help to identify more women with forearm fractures. Thus, physicians finding a T-score in the osteopenic range are likely to not initiate treatment even though most forearm fractures arise from this group.
By adding a measurement of cortical porosity at the ultra distal radius, a subset of women with ultradistal radius or FN osteopenia were found to contribute to the burden of fractures. Thus, in women found to have osteopenia, it may be appropriate to also measure porosity at the ultra distal radius. Finding high porosity, which compromises bone strength out of proportion to the modest reduction in BMD that characterizes osteopenia, identifies individuals in need of treatment who would not be identified otherwise.
This work was confirmed by studying the association between fractures and porosity measured at the proximal femur. Ahmed et al. [58] reported that each SD higher porosity at this location was also associated with fracture independent of FN BMD (OR 1.39; 95% CI, 1.11 to 1.74) and Fracture Risk Assessment (FRAX) score (OR, 1.58; 95% CI, 1.27 to 1.97) in all women studied. Porosity was also associated with fracture independent of FRAX score in those with normal FN BMD (OR 1.88; 95% CI, 1.21 to 2.94), osteopenia (OR, 1.40; 95% CI, 1.06 to 1.85), but not significantly in those with osteoporosis (OR, 1.48; 95% CI, 0.68 to 3.23). Of the 211 fracture cases, only 18 women (9%) were identified using FN BMD T-score <-2.5, 45 women (21%) using FRAX threshold >20%, whereas porosity >80th percentile identified 61 women (29%). Porosity identified 26% additional women with fractures than identified by the osteoporosis threshold and 21% additional women with fractures than by this FRAX threshold.
The transition from cortical to trabecular bone is gradual so that it is not possible to accurately identify an edge that corresponds to the endocortical surface [39]. Quantifying the transitional zone is important because it is a way of avoiding erroneously apportioning the fragmented cortex and the pores that fragmented it into the medullary (trabecular) compartment. Failure to retain the fragments and porosity as part of the transitional zone produces several errors.
It underestimates the age-related and menopause-related increase in cortical porosity because porosity is erroneously 'seen' as part of the medullary void volume. It 'looks like' the medullary canal is expanding; the morphology should not be 'seen' in this way because the intracortical 'pores' are not medullary void, they are voids produced by cavitation of the cortex. In addition, the age-related and menopause-related decline in trabecular number and thickness is underestimated because cortical fragments in the transitional zone are 'seen' as part of the medullary canal, which falsely elevates trabecular density in old age and so blunts the diminution in trabecular bone across age and after menopause. Both of these errors underestimate fracture risk. Moreover, the age-related and menopause-related loss of cortical mineralized bone matrix volume is overestimated because cortical fragments erroneously allocated to the 'medullary' canal are not quantified as being part of the cortical bone in older persons [25].
The best image resolution achievable in vivo using HR-pQCT is ~120 microns which precludes quantification of most pores because over 60% of pores are less than 100 microns in diameter [39]. Direct measurements of cortical bone water using deuterium oxide or dehydration experiments report a void volume of 15% to 40% [5960616263]. The low porosities reported in most studies are incompatible with these direct measurements, and with the provision of a vascular supply [64656667]. Non-threshold-based approach to quantify porosity avoids exclusion of voxels containing mineralized matrix and void. The presence of mineralized matrix increases photon attenuation so threshold based image analysis excludes that voxel with its void volume and so underestimates porosity [3839].
Cortical porosity in adulthood is the net result of the porosity achieved during growth, constituted mainly by the Haversian and Volkmann canals, and the subsequent increase in porosity produced by age related intracortical remodelling initiated upon these canals surfaces. Excavation of bone matrix enlarges the canals focally and produces coalescent and giant pores in cross section as age advances [686970]. Prospective studies are needed to determine whether a measurement of porosity and other microarchitectural traits will identifying women sustaining fractures who then can be targeted for therapy before the fracture occurs [7172]. Studies are also needed to determine whether measurement of porosity will help to determine whether treatment is successful by reducing porosity, whether the reduction in porosity explained the fracture risk reduction, and whether treatment fails to reduce porosity and the persisting porosity accounts for continued fractures despite compliance with therapy [73747576].
Figures and Tables
Fig. 1
Right panel: trabecular bone is configured as thin plates of mineralized bone matrix enveloped by a large surface area which facilitates initiation of bone remodelling. Left panel: cortical bone is configured with a larger volume of mineralized bone matrix enveloped by the periosteal, intracortical and endocortical surfaces. The smaller surface area relative to the large matrix volume results in the cortical matrix being less accessible to being remodelled (see text).
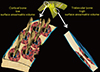
Fig. 2
(A) The surfaces of bone. Cortical bone volume comprises both the mineralized bone matrix volume and the void volume formed largely by the intracortical canals traversing it. Thus, cortical bone matrix volume is 'inside' the periosteal surface and 'outside' the intracortical and endocortical surfaces upon which remodelling is initiated. Trabecular bone is contained within the trabecular surfaces which are contiguous with the endocortical surface. (B) Remodling upon the intracortical surface enlarges the canals so that with time they coalesce forming giant pores in cross section with fragment the cortex so it is trabecularized. By failing to maintain the anatomical location of the cortical compartment and medullar compartment (broken white line) errors occur in ascribing the matrix and void volumes to the correct location. Including a transitional zone helps to avoid these errors (see text).

References
1. Albright F. Osteoporosis. Ann Intern Med. 1947; 27:861–882.
2. Eastell R, Mosekilde L, Hodgson SF, Riggs BL. Proportion of human vertebral body bone that is cancellous. J Bone Miner Res. 1990; 5:1237–1241.
3. Rockoff SD, Sweet E, Bleustein J. The relative contribution of trabecular and cortical bone to the strength of human lumbar vertebrae. Calcif Tissue Res. 1969; 3:163–175.
4. Hui SL, Slemenda CW, Johnston CC, Appledorn CR. Effects of age and menopause on vertebral bone density. Bone Miner. 1987; 2:141–146.
5. Hesp R, Arlot ME, Edouard C, Bradbeer JN, Meunier PJ, Reeve J. Iliac trabecular bone formation predicts radial trabecular bone density changes in type 1 osteoporosis. J Bone Miner Res. 1991; 6:929–935.
6. Delmas PD, Fontanges E, Duboeuf F, Boivin G, Chavassieux P, Meunier PJ. Comparison of bone mass measured by histomorphometry on iliac biopsy and by dual photon absorptiometry of the lumbar spine. Bone. 1988; 9:209–213.
7. Eastell R, Riggs BL, Wahner HW, O'Fallon WM, Amadio PC, Melton LJ 3rd. Colles' fracture and bone density of the ultradistal radius. J Bone Miner Res. 1989; 4:607–613.
8. Eastell R, Wahner HW, O'Fallon WM, Amadio PC, Melton LJ 3rd, Riggs BL. Unequal decrease in bone density of lumbar spine and ultradistal radius in Colles' and vertebral fracture syndromes. J Clin Invest. 1989; 83:168–174.
9. Wahner HW. Single- and dual-photon absorptiometry in osteoporosis and osteomalacia. Semin Nucl Med. 1987; 17:305–315.
10. Melton LJ 3rd, Atkinson EJ, O'Fallon WM, Wahner HW, Riggs BL. Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res. 1993; 8:1227–1233.
11. Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone. 2002; 30:807–809.
12. Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983; 72:1396–1409.
13. Pouilles JM, Tremollieres F, Ribot C. Spine and femur densitometry at the menopause: are both sites necessary in the assessment of the risk of osteoporosis? Calcif Tissue Int. 1993; 52:344–347.
14. Seeman E. Structural basis of growth-related gain and age-related loss of bone strength. Rheumatology (Oxford). 2008; 47:Suppl 4. iv2–iv8.
15. Parfitt AM, Travers R, Rauch F, Glorieux FH. Structural and cellular changes during bone growth in healthy children. Bone. 2000; 27:487–494.
16. Rauch F, Travers R, Glorieux FH. Intracortical remodeling during human bone development: a histomorphometric study. Bone. 2007; 40:274–280.
17. Schnitzler CM, Mesquita JM, Pettifor JM. Cortical bone development in black and white South African children: iliac crest histomorphometry. Bone. 2009; 44:603–611.
18. Cadet ER, Gafni RI, McCarthy EF, McCray DR, Bacher JD, Barnes KM, et al. Mechanisms responsible for longitudinal growth of the cortex: coalescence of trabecular bone into cortical bone. J Bone Joint Surg Am. 2003; 85-A:1739–1748.
19. Enlow DH. A study of the post-natal growth and remodeling of bone. Am J Anat. 1962; 110:79–101.
20. Enlow DH. Principles of bone remodeling: an account of post-natal growth and remodeling processes in long bones and the mandible. Springfield: Thomas Books;1963.
21. Wang Q, Ghasem-Zadeh A, Wang XF, Iuliano-Burns S, Seeman E. Trabecular bone of growth plate origin influences both trabecular and cortical morphology in adulthood. J Bone Miner Res. 2011; 26:1577–1583.
22. Bala Y, Bui QM, Wang XF, Iuliano S, Wang Q, Ghasem-Zadeh A, et al. Trabecular and cortical microstructure and fragility of the distal radius in women. J Bone Miner Res. 2015; 30:621–629.
23. Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res. 2010; 25:1521–1526.
24. Keshawarz NM, Recker RR. Expansion of the medullary cavity at the expense of cortex in postmenopausal osteoporosis. Metab Bone Dis Relat Res. 1984; 5:223–228.
25. Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010; 375:1729–1736.
26. Bergstrom U, Bjornstig U, Stenlund H, Jonsson H, Svensson O. Fracture mechanisms and fracture pattern in men and women aged 50 years and older: a study of a 12-year population-based injury register, Umea, Sweden. Osteoporos Int. 2008; 19:1267–1273.
27. Hedstrom EM, Svensson O, Bergstrom U, Michno P. Epidemiology of fractures in children and adolescents. Acta Orthop. 2010; 81:148–153.
28. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000; 15:721–739.
29. Havill LM, Allen MR, Harris JA, Levine SM, Coan HB, Mahaney MC, et al. Intracortical bone remodeling variation shows strong genetic effects. Calcif Tissue Int. 2013; 93:472–480.
30. Havill LM, Allen MR, Bredbenner TL, Burr DB, Nicolella DP, Turner CH, et al. Heritability of lumbar trabecular bone mechanical properties in baboons. Bone. 2010; 46:835–840.
31. Bjornerem A, Bui M, Wang X, Ghasem-Zadeh A, Hopper JL, Zebaze R, et al. Genetic and environmental variances of bone microarchitecture and bone remodeling markers: a twin study. J Bone Miner Res. 2015; 30:519–527.
32. Mikkola TM, Sipila S, Rantanen T, Sievanen H, Suominen H, Kaprio J, et al. Genetic and environmental influence on structural strength of weight-bearing and non-weight-bearing bone: a twin study. J Bone Miner Res. 2008; 23:492–498.
33. Lips P, Courpron P, Meunier PJ. Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res. 1978; 26:13–17.
34. Vedi S, Compston JE, Webb A, Tighe JR. Histomorphometric analysis of dynamic parameters of trabecular bone formation in the iliac crest of normal British subjects. Metab Bone Dis Relat Res. 1983; 5:69–74.
35. Bjornerem A, Ghasem-Zadeh A, Bui M, Wang X, Rantzau C, Nguyen TV, et al. Remodeling markers are associated with larger intracortical surface area but smaller trabecular surface area: a twin study. Bone. 2011; 49:1125–1130.
36. Foldes J, Parfitt AM, Shih MS, Rao DS, Kleerekoper M. Structural and geometric changes in iliac bone: relationship to normal aging and osteoporosis. J Bone Miner Res. 1991; 6:759–766.
37. Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res. 1997; 12:498–508.
38. Zebaze R, Ghasem-Zadeh A, Mbala A, Seeman E. A new method of segmentation of compact-appearing, transitional and trabecular compartments and quantification of cortical porosity from high resolution peripheral quantitative computed tomographic images. Bone. 2013; 54:8–20.
39. Zebaze R, Seeman E. Cortical bone: a challenging geography. J Bone Miner Res. 2015; 30:24–29.
40. Ruff CB, Hayes WC. Sex differences in age-related remodeling of the femur and tibia. J Orthop Res. 1988; 6:886–896.
41. Bouxsein ML. Determinants of skeletal fragility. Best Pract Res Clin Rheumatol. 2005; 19:897–911.
42. Holzer G, von Skrbensky G, Holzer LA, Pichl W. Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res. 2009; 24:468–474.
43. Nawathe S, Nguyen BP, Barzanian N, Akhlaghpour H, Bouxsein ML, Keaveny TM. Cortical and trabecular load sharing in the human femoral neck. J Biomech. 2015; 48:816–822.
44. Lotz JC, Cheal EJ, Hayes WC. Stress distributions within the proximal femur during gait and falls: implications for osteoporotic fracture. Osteoporos Int. 1995; 5:252–261.
45. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008; 23:392–399.
46. Schaffler MB, Burr DB. Stiffness of compact bone: effects of porosity and density. J Biomech. 1988; 21:13–16.
47. Rice JC, Cowin SC, Bowman JA. On the dependence of the elasticity and strength of cancellous bone on apparent density. J Biomech. 1988; 21:155–168.
48. Martin RB, Ishida J. The relative effects of collagen fiber orientation, porosity, density, and mineralization on bone strength. J Biomech. 1989; 22:419–426.
49. Burr D. Microdamage and bone strength. Osteoporos Int. 2003; 14:Suppl 5. S67–S72.
50. Diab T, Vashishth D. Effects of damage morphology on cortical bone fragility. Bone. 2005; 37:96–102.
51. Martin RB, Burr DB. The microscopic structure of bone. New York: Raven Press;1989.
52. Yeni YN, Brown CU, Wang Z, Norman TL. The influence of bone morphology on fracture toughness of the human femur and tibia. Bone. 1997; 21:453–459.
53. Granke M, Grimal Q, Saied A, Nauleau P, Peyrin F, Laugier P. Change in porosity is the major determinant of the variation of cortical bone elasticity at the millimeter scale in aged women. Bone. 2011; 49:1020–1026.
54. Bala Y, Zebaze R, Ghasem-Zadeh A, Atkinson EJ, Iuliano S, Peterson JM, et al. Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res. 2014; 29:1356–1362.
55. Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004; 164:1108–1112.
56. Sanders KM, Nicholson GC, Watts JJ, Pasco JA, Henry MJ, Kotowicz MA, et al. Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone. 2006; 38:694–700.
57. Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004; 34:195–202.
58. Ahmed LA, Shigdel R, Joakimsen RM, Eldevik OP, Eriksen EF, Ghasem-Zadeh A, et al. Measurement of cortical porosity of the proximal femur improves identification of women with nonvertebral fragility fractures. Osteoporos Int. 2015; 26:2137–2146.
59. Chappard C, Bensalah S, Olivier C, Gouttenoire PJ, Marchadier A, Benhamou C, et al. 3D characterization of pores in the cortical bone of human femur in the elderly at different locations as determined by synchrotron micro-computed tomography images. Osteoporos Int. 2013; 24:1023–1033.
60. Biltz RM, Pellegrino ED. The chemical anatomy of bone. I. A comparative study of bone composition in sixteen vertebrates. J Bone Joint Surg Am. 1969; 51:456–466.
61. Mueller KH, Trias A, Ray RD. Bone density and compostiton. Age-related and pathological changes in water and mineral content. J Bone Joint Surg Am. 1966; 48:140–148.
62. Smith JW. Observations on the water Content of bone. J Bone Joint Surg Br. 1964; 46:553–562.
63. Techawiboonwong A, Song HK, Leonard MB, Wehrli FW. Cortical bone water: in vivo quantification with ultrashort echo-time MR imaging. Radiology. 2008; 248:824–833.
64. Tjong W, Kazakia GJ, Burghardt AJ, Majumdar S. The effect of voxel size on high-resolution peripheral computed tomography measurements of trabecular and cortical bone microstructure. Med Phys. 2012; 39:1893–1903.
65. Geusens P, Chapurlat R, Schett G, Ghasem-Zadeh A, Seeman E, de Jong J, et al. High-resolution in vivo imaging of bone and joints: a window to microarchitecture. Nat Rev Rheumatol. 2014; 10:304–313.
66. Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010; 25:882–890.
67. Nishiyama KK, Macdonald HM, Moore SA, Fung T, Boyd SK, McKay HA. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study. J Bone Miner Res. 2012; 27:273–282.
68. Jordan GR, Loveridge N, Bell KL, Power J, Rushton N, Reeve J. Spatial clustering of remodeling osteons in the femoral neck cortex: a cause of weakness in hip fracture? Bone. 2000; 26:305–313.
69. Bell KL, Loveridge N, Reeve J, Thomas CD, Feik SA, Clement JG. Super-osteons (remodeling clusters) in the cortex of the femoral shaft: influence of age and gender. Anat Rec. 2001; 264:378–386.
70. Pfeiffer S, Crowder C, Harrington L, Brown M. Secondary osteon and Haversian canal dimensions as behavioral indicators. Am J Phys Anthropol. 2006; 131:460–468.
71. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005; 90:6508–6515.
72. Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res. 2010; 25:2572–2581.
73. Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007; 22:425–433.
74. Bala Y, Chapurlat R, Cheung AM, Felsenberg D, LaRoche M, Morris E, et al. Risedronate slows or partly reverses cortical and trabecular microarchitectural deterioration in postmenopausal women. J Bone Miner Res. 2014; 29:380–388.
75. Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res. 2010; 25:2558–2571.
76. Zebaze RM, Libanati C, Austin M, Ghasem-Zadeh A, Hanley DA, Zanchetta JR, et al. Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone. 2014; 59:173–179.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download