Abstract
Background
Hes6 is a transcriptional regulator that induces transcriptional activation by binding to transcription repressor Hes1 and suppressing its activity. Hes6 is controlled by the ubiquitin-proteosome-mediated degradation system. Here we investigated the sumoylation of Hes6 and its functional role in its rhythmic expression.
Methods
Hes6, SUMO, and ubiquitin were transfected into HeLa cells and the expression pattern was observed by Western blot and immunoprecipitation. To confirm the effect of sumoylation on the rhythmic expression of Hes6, we generated mouse Hes6 promoter-driven GFP-Hes6 fusion constructs and expressed these constructs in NIH 3T3 cells.
Results
Overexpression of SUMO led to sumoylation of Hes6 at both lysine 27 and 30. Protein stability of Hes6 was decreased by sumoylation. Moreover, expression of a Hes6 sumoylation-defective mutant, the 2KR (K27/30R) mutant, or co-expression of SUMO protease SUSP1 with native Hes6, strongly reduced ubiquitination. In addition, sumoylation was associated with both the rhythmic expression and transcriptional regulation of Hes6. Wild type Hes6 showed oscillatory expression with about 2-hour periodicity, whereas the 2KR mutant displayed a longer period. Furthermore, sumoylation of Hes6 derepressed Hes1-induced transcriptional repression.
Hes genes are mammalian homologues of Drosophila hairy and enhancer of split, which encode the basic helix-loop-helix (bHLH) transcription factors and play important roles in development [123]. Although Hes factors have the bHLH domain, they preferentially bind to the N-boxes (CACNAG) rather than to the E-box (CANNTG) [45]. Among Hes family members, Hes1 and Hes5 are regulated by Notch signaling and repress downstream target gene transcription, resulting in inhibition of neuronal differentiation [67]. In contrast, Hes6 acts as a positive regulator and promotes cell differentiation. Hes6 antagonizes the action of Hes1 by repressing its transcriptional activity, but it does not directly bind to DNA alone [89].
A remarkable feature of the Hes family members Hes1 and Hes7 is rhythmic expression with a period of about 2 hours [101112]. Real-time imaging analysis showed that Hes1 dynamically oscillates in fibroblasts as well as in neural progenitors [1314]. This rhythmic expression is regulated by negative feedback and the instability of the gene products [151617]. Hes1 represses its own transcription by directly binding to the N-box of the Hes1 promoter through negative feedback [18]. In this feedback pathway, ubiquitin-mediated protein degradation plays a pivotal role in maintaining rhythmic expression. Treatment with a proteasome inhibitor leads to stabilization of the Hes1 protein and eventually blocks the oscillatory transcription of Hes [1517]. Also, the C-terminal WRPW (Trp-Arg-Pro-Trp) motif of Hes6 is important for proteasome-dependent degradation of Hes6 itself [19]. The overall protein expression level of the WRPW motif-deleted Hes6 mutant is increased when compared to wild type Hes6.
Sumoylation is a posttranslational modification that regulates protein functions such as transcriptional regulation, cellular localization, and protein-protein interaction [20]. A recent report demonstrates that sumoylation of Hes1 regulates cell survival [21]. Sumoylation increases the stability of the Hes1 protein and induces transcriptional repression of target genes by Hes1. However, Hes5 is not a substrate for sumoylation [21]. Furthermore, sumoylation is closely related to ubiquitination. SUMO and ubiquitin share structural characteristics and conjugation mechanisms to particular lysine residues of their target proteins [22]. Although sumoylation is not directly linked to target protein degradation, it can control protein stability via ubiquitin-mediated proteolysis [23].
In the present study, we investigated sumoylation of Hes6 and its functional roles. Our data demonstrate that sumoylation of Hes6 promotes ubiquitin-dependent protein turnover and sumoylated Hes6 affects its own rhythmic expression with a period of about 2 hours. Moreover, Hes6 sumoylation derepressed Hes1-dependent transcriptional repression by attenuating the N-box binding of Hes1/Hes6 complex.
HeLa and NIH 3T3 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA) at 37℃ with 9% CO2. For transient transfection, cells were seeded at 105 cells/well in 12-well plates. After overnight incubation, expression constructs were transfected using Lipofectamin Plus reagent (Invitrogen) according to the manufacturer's protocol. For protein stability and ubiquitination studies, cells were treated with cycloheximide (CHX) at 30 µg/mL and MG132 at 25 µM for indicated time periods.
Hes6 expression constructs were kindly provided by Dr. Sun (Korea University, Seoul, Korea). The point mutants were generated using the QuickChange site-directed mutagenesis as directed by the manufacturer's protocol (Stratagene, La Jolla, CA, USA). The polymerase chain reaction (PCR) primers used for the site-directed mutagenesis were as follows: for the K27R mutant, 5'-GCACGGGGGGACCGCA GGGCCCGGAAG CCCC-3'; for the K30R mutant, 5'-GGGACCGCAAGGCCCGGAGG CCCCTGGTGGAG-3'; for the K35R mutant, 5'-GCCCCTGGTGGAGAGGAAGCGACG CGCACGG-3'; for the K36R mutant, 5'-GCCCCTGGTGGAGAAGAGGCGACGCGCAC GG-3'; for the K60R mutant, 5'-GGTACCGAGGTGCAGGCCAGGCTAGAGAACGCCG-3'; for the 2KR (K27/30R) double mutant, 5'-CGGGGGGACCGCAGGG CCCGGAGGC CCCTGGTGG-3'. The mutagenesis was confirmed by DNA sequencing.
Cells were harvested in radioimmunoprecipitation assay buffer (50 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [pH 7.4], 150 mmol/L NaCl, 1% NP-40, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L ethylene glycol tetraacetic acid, 1 mmol/L phenylmethanesulfonylfluoride, 0.5% sodium deoxycholate, 1 mmol/L NaF, 1 mmol/L Na3VO4 and 1×protease inhibitor cocktail [Sigma, St. Louis, MO, USA]) and centrifuged for 20 minutes at 4℃. Equal amounts of total protein were incubated with 2 µg anti-Hes6 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-Hes1 (AbFrontier, Seoul, Korea) antibodies for 1.5 hours at 4℃ and then the samples were incubated with the protein G-Sepharose bead slurry for 1 hour at 4℃. The final immune complexes were analyzed by Western blot.
For Western blot analyses, samples were resolved on 10% or 12% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Immobilon P, Millipore, Bedford, MA, USA). Target proteins were detected with anti-Hes6 (anti-Hes6 antiserum was kindly provided by Dr. Sun), anti-SUMO1, anti-SUMO3 (Invitrogen), anti-Hes1 (Abfrontier), anti-HDAC3, anti-HA, or anti-actin (Santa Cruz Biotechnology) antibodies. Horseradish peroxidase-conjugated secondary antibodies were purchased from Jackson Immunoresearch Laboratories (West Grove, PA, USA) and the immune complexes were visualized with an enhanced chemiluminescent detection kit (Pierce, Rockford, IL, USA).
Chromatin immunoprecipitation (ChIP) assays were performed according to the manufacturer's instructions (Millipore). Each cross-linked sample was immunoprecipitated using anti-Hes1 (Abfrontier), anti-Hes6 (antiserum), anti-HDAC3 (Santa Cruz Biotechnology), or anti-histone H3 (acetyl K9, Abcam, Cambridge, MA, USA) antibodies and the DNA was purified by phenol/chloroform extraction followed by ethanol precipitation. For PCR, the following primers were used for the Hes1 N-box, 5'-TCCTTTTGATTGACGTTGTAGC-3' and 5'-GCACTATTCCAGGACCAAGG-3'.
To examine whether Hes6 is a substrate of sumoylation, Hes6 was co-transfected with RFP-fused SUMO paralogues (SUMO1 or SUMO3) into HeLa cells. In the presence of SUMO1 or SUMO3, sumoylation of Hes6 was observed and was enhanced by the E2-conjugating enzyme, Ubc9 (Fig. 1A). However, the Hes6 sumoylation band was not detected when co-expressed with ΔSUMO, a sumoylation defective mutant lacking the C-terminal motif (data not shown). To confirm whether this band was indeed a sumoylated form, Hes6 was co-expressed with RFP-SUMO paralogues and Ubc9 and then immunoprecipitated using anti-Hes6 antibodies. A slow migrating band of Hes6 was clearly detected by anti-Hes6 antibodies and SUMO1- or SUMO3-modified bands were detected using specific antibodies (Fig. 1B). Thus, these data suggest that Hes6 is covalently modified by either SUMO1 or SUMO3.
Next, to determine which lysine residues of Hes6 are targets of sumoylation, we next examined which lysine residues of Hes6 are targets for sumoylation. Within the bHLH domain of Hes6, there were five lysine residues positioned at K27, K30, K35, K36, and K60. We generated point mutants of Hes6 with arginine substituted for lysine residues. As shown in Fig. 2A, sumoylation of K27R or K30R single mutant was reduced in comparison with wild type Hes6, while the other mutants (K35R, K36R, or K60R mutants) did not show any decrease in sumoylation. Moreover, Hes6 mutations at both K27R and K30R (2KR mutant) completely abolished sumoylation (Fig. 2B). These data indicate that both lysine 27 and 30 of Hes6 are the target sites for sumoylation.
Different kinds of posttranslational modifications including sumoylation and ubiquitination are closely related to each other [22]. For instance, sumoylation blocks ubiquitin-dependent protein degradation of IκBα [24]. On the other hand, sumoylation of BMAL1 promotes its ubiquitination-mediated proteasomal degradation [25]. Because Hes6 also is degraded in an ubiquitin-dependent manner [19], we tested the relationship between Hes6 sumoylation and ubiquitination. First, we examined the protein stability of wild type or 2KR mutant Hes6. HeLa cells were transfected with either wild type Hes6 or the 2KR mutant and treated with CHX, an inhibitor of protein synthesis, for the indicated time periods. As shown in Fig. 3A, protein stability of wild type Hes6 was rapidly diminished, while the 2KR mutant showed much higher stability. We then examined the effect of sumoylation on ubiquitin-dependent Hes6 degradation. Wild type or the 2KR mutant Hes6 was co-transfected with HA-tagged ubiquitin in the presence or absence of RFP-SUMO. By Western blot, moderate ubiquitination of wild type Hes6 was detected by co-expression of HA-ubiquitin and further enhanced by co-expression of SUMO. Ubiquitination of wild type Hes6 was significantly decreased by SUMO protease SUSP1 (Fig. 3B). However, co-expression of either HA-ubiquitin alone or HA-ubiquitin together with SUMO failed to induce strong ubiquitination of the 2KR Hes6 mutant (Fig. 3B). Thus, these results demonstrate that sumoylation of Hes6 appears to accelerate its own ubiquitination leading to degradation via 26S proteasome pathway.
Previous studies demonstrate that gene expression of Hes family members such as Hes1 and Hes7 oscillate with a period of about 2 hours. This rhythmic expression plays an important role in the regulation of timely events in development processes [151617]. In circadian regulation, it has been reported previously that sumoylation of BMAL1 is critical for the oscillation of downstream clock genes as well as BMAL1 itself [2526]. Therefore, we examined whether sumoylation of Hes6 is involved in its rhythmic expression. We generated expression constructs of GFP-fused wild type or 2KR mutant Hes6 under the control of Hes6 promoter (Fig. 4A). These constructs were transfected into NIH 3T3 cells and the cells were incubated with serum to induce rhythmic expression of the Hes6 promoter. As shown in Fig. 4, 2-hour rhythmic expression of wild type Hes6 was induced by 50% horse serum shock (Fig. 4B, upper panel of Western blot). However, serum treatment failed to induce oscillation in the 2KR mutant Hes6 (Fig. 4B, lower panel of Western blot). Quantitation of band intensity of either wild type or the 2KR mutant Hes6 are shown in the bottom of Fig. 4B.
Hes6 is a positive regulator that derepresses Hes1 activity and thereby activates transcription of downstream genes [8]. We hypothesized that sumoylation might be important for the effect of Hes6 on Hes1-dependent transcriptional repression. As expected, Hes1-mediated transcriptional repression was relieved by Hes6 in a dose-dependent manner (Fig. 5A). Moreover, as shown in Fig. 5B, wild type Hes6 almost completely derepressed Hes1-induced transcriptional attenuation, while the 2KR mutant Hes6 exhibited partial derepression (Fig. 5B). To elucidate the molecular mechanism underlying the effect of Hes6 sumoylation on Hes1-mediated transcriptional repression, we examined interactions between Hes1 and Hes6 or Hes1 and DNA by immunoprecipitation or ChIP assay, respectively. Unexpectedly, Hes1 exhibited increased interaction with the 2KR mutant compared to wild type Hes6 (Fig. 5C). Moreover, in ChIP assays using either Hes1 or Hes6 antibodies, the Hes1/2KR Hes6 complex exhibited enhanced N-box binding than the Hes1/wild type Hes6 (Fig. 5D). Furthermore, histone H3 acetylation was decreased by co-expression of the 2KR mutant Hes6. HDAC recruitment to the N-box region was significantly induced by the 2KR mutant Hes6 (Fig. 5C, D). Taken together, these results demonstrate that sumoylation of Hes6 may play an important role in controlling Hes1 transcriptional activity by regulating interaction between Hes6 and Hes1, N-box binding of the Hes1/Hes6 complex, and recruitment of co-regulators to the promoter.
Hes genes belong to the basic bHLH transcription factor family and play an important role in development and cell differentiation [27]. Among the Hes family members, Hes1 functions as a negative regulator that inhibits target gene transcription and suppresses neural differentiation, whereas Hes6 promotes cell differentiation by repressing the transcriptional activity of Hes1 [78]. However, the molecular mechanism of Hes6 in transcriptional regulation is largely unknown. In the present study, we investigated the effect of posttranslational modifications, especially sumoylation, on Hes6 function. We found that Hes6 is a substrate for sumoylation, which controlled the ubiquitin-dependent degradation of Hes6 itself. Moreover, Hes6 sumoylation was associated with the interaction with Hes1 and recruitment of co-factors for transcriptional regulation.
Recently, it was reported that Hes1 sumoylation increases the repressive effect of Hes1, but shows a partial effect on the regulation of gene expression by Hes1 [21]. Indeed, sumoylation also induces the transcriptional activity of Hes6 similar to Hes1 sumoylation. Luciferase reporter assays using the N-box containing Hes1 promoter showed that wild type Hes6 significantly derepressed Hes1-induced transcriptional suppression compared to the 2KR mutant (Fig. 5B). Moreover, Hes6 sumoylation reduced both protein interaction between Hes1 and Hes6 and the DNA binding ability of Hes1/Hes6 complex to the N-box element (Fig. 5C, D). Although sumoylation was known to induce transcriptional activity of both Hes1 and Hes6, sumoylation has the opposite effect on protein stability of Hes1 or Hes6. Sumoylation stabilized Hes1 [21], whereas the protein stability of Hes6 was decreased by sumoylation-mediated promotion of Hes6 ubiquitination (Fig. 3). These results raise a possibility that Hes6 sumoylation promotes its degradation and simultaneously releases Hes1 from the N-box region.
Hes family members generate rhythmicity of about 2-hour periods and the dynamic regulation of Hes stability is controlled by its own transcriptional regulation as well as by ubiquitin-dependent proteolysis [151617]. A recent study demonstrates that circadian rhythmic expression of Hes1 and Hes6 are under the control of a molecular circadian clock in the mouse liver [28]. Hes1 or Hes6 mRNA expression showed opposite rhythmicity in wild type mice and knockout of both Per1 and Per2 destroyed the rhythmic expression pattern. In addition, transcription of Hes6 was directly activated by CLOCK/BMAL1 heterodimers, transcription activators of the circadian clock. However, oscillatory expression with 2-hour periods of Hes6 had not been defined. Using wild type or 2KR mutant of Hes6 constructs under the control of Hes6 promoter, we identified the 2-hour rhythmic expression of Hes6 and the effect of sumoylation on the Hes6 expression (Fig. 4). Interestingly, wild type Hes6 exhibited rhythmic expression with about 2-hour periods like the other Hes family members. The 2KR mutant of Hes6 also showed a rhythmic expression profile, but the period was significantly lengthened compared to wild type Hes6 (Fig. 4B). The lengthened period in the 2KR mutant seemed to be caused by enhanced protein stability resulting from lack of sumoylation-dependent protein degradation, which is mediated by ubiquitination (Fig. 3B). Thus, these results suggest that posttranslational modifications including sumoylation and ubiquitination may play an important role in the rhythmic expression of Hes6 with about 2-hour periods.
In summary, we showed that Hes6 is sumoylated at both lysine 27 and 30 and sumoylation promotes ubiquitin-dependent degradation of Hes6. Moreover, Hes6 sumoylation may be critical for the regulation of Hes1-mediated transcriptional repression as well as the oscillatory expression of Hes6 itself.
Figures and Tables
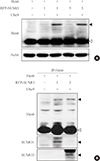 | Fig. 1Sumoylation of Hes6. (A) HeLa cells were co-transfected with Hes6 and RFP-SUMO paralogues (SUMO1 or SUMO3) in the presence or absence of Ubc9. Samples were analyzed by Western blot using anti-Hes6 antibodies. (B) Hes6, RFP-SUMO, and Ubc9 were co-expressed in cells and the cell extracts were subjected to immunoprecipitation followed by Western blot using anti-Hes6, anti-SUMO1, or anti-SUMO3 antibodies. Arrowheads indicate the sumoylated Hes6 (black) or native Hes6 (white) band. |
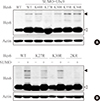 | Fig. 2Hes6 is sumoylated at lysine residues 27 and 30. (A) Proteins were extracted from HeLa cells transfected with Hes6 constructs encoding wild type (WT) or its Lys-Arg mutants. Western blot was performed with the indicated antibodies. (B) Cells were transfected with Hes6 constructs encoding WT, the single mutants (K27R or K30R), or the 2KR (K27/30R) double mutant together with SUMO and Ubc9 and analyzed by Western blot using anti-Hes6 antibodies. |
 | Fig. 3Sumoylation promotes ubiquitination of Hes6. (A) HeLa cells expressing wild type (WT) Hes6 or the 2KR (K27/30R) double mutant were treated with cychloheximide (CHX, 30 µg/mL) for indicated time periods. The protein abundance of Hes6 was analyzed by Western blot using anti-Hes6 antibodies and then quantitated by densitometer. (B) Cells were co-transfected with WT Hes6 or the 2KR mutant and HA-tagged ubiquitin (Ub) in the presence or absence of RFP-SUMO and SUSP1 and incubated for 5 hours with 25 µM MG132. Cell extracts were analyzed by immunoprecipitation followed by Western blot using anti-HA, anti-SUMO, or anti-Hes6 antibodies. |
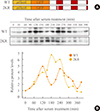 | Fig. 4Sumoylation regulates rhythmic expression of Hes6. (A) The schematic diagram of GFP-fused wild type (WT) or the 2KR (K27/30R) mutant Hes6 under the control of the Hes6 promoter. (B) NIH 3T3 cells were transfected with GFP-Hes6 constructs encoding WT or the 2KR mutant. After serum treatment (t=0), Hes6 protein levels were examined every 30 minutes. |
 | Fig. 5Effects of Hes6 sumoylation on Hes1-induced transcriptional suppression. (A) HeLa cells were co-transfected with pHes1-luciferase reporter, Hes1 (20 ng), and increasing amounts of Hes6 (50, 100, 200, and 300 ng). After overnight incubation, the bioluminescence was measured using a luminometer. Each value is the mean±SEM of three independent experiments. (B) Cells were co-transfected with the pHes1-luciferase reporter, Hes1, and Hes6 (wild type [WT] or the 2KR [K27/30R] mutant). Each value is the mean±SEM of three independent experiments (P<0.001). (C) Cells were co-transfected with Hes1 and Hes6 and the cell lysates were immunoprecipitated using anti-Hes1 antibodies. (D) Chromatin was extracted from HeLa cells transfected with Hes1 and Hes6 constructs encoding WT or the 2KR mutant. Chromatin immunoprecipitation (ChIP) assays were performed with the indicated antibodies. aP<0.001 vs. Hes1 expression; bP<0.001. |
ACKNOWLEDGMENTS
This work was supported by National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013M3C7A1056731 and NRF-2014R1A 2A1A01003680), and the BK21 Plus program through the National Research Foundation of Korea funded by the Ministry of Education (10Z20130012420). BioScience Writers edited the manuscript.
References
1. Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002; 3:517–530.
2. Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003; 39:13–25.
3. Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007; 134:1243–1251.
4. Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992; 6:2620–2634.
5. Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, et al. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci U S A. 1997; 94:5355–5360.
6. Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995; 377:355–358.
7. Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999; 18:2196–2207.
8. Bae S, Bessho Y, Hojo M, Kageyama R. The bHLH gene Hes6, an inhibitor of Hes1, promotes neuronal differentiation. Development. 2000; 127:2933–2943.
9. Koyano-Nakagawa N, Kim J, Anderson D, Kintner C. Hes6 acts in a positive feedback loop with the neurogenins to promote neuronal differentiation. Development. 2000; 127:4203–4216.
10. Harima Y, Imayoshi I, Shimojo H, Kobayashi T, Kageyama R. The roles and mechanism of ultradian oscillatory expression of the mouse Hes genes. Semin Cell Dev Biol. 2014; 34:85–90.
11. Pascoal S, Carvalho CR, Rodriguez-Leon J, Delfini MC, Duprez D, Thorsteinsdottir S, et al. A molecular clock operates during chick autopod proximal-distal outgrowth. J Mol Biol. 2007; 368:303–309.
12. Schwendinger-Schreck J, Kang Y, Holley SA. Modeling the zebrafish segmentation clock's gene regulatory network constrained by expression data suggests evolutionary transitions between oscillating and nonoscillating transcription. Genetics. 2014; 197:725–738.
13. Masamizu Y, Ohtsuka T, Takashima Y, Nagahara H, Takenaka Y, Yoshikawa K, et al. Real-time imaging of the somite segmentation clock: revelation of unstable oscillators in the individual presomitic mesoderm cells. Proc Natl Acad Sci U S A. 2006; 103:1313–1318.
14. Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008; 58:52–64.
15. Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, et al. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002; 298:840–843.
16. Hirata H, Bessho Y, Kokubu H, Masamizu Y, Yamada S, Lewis J, et al. Instability of Hes7 protein is crucial for the somite segmentation clock. Nat Genet. 2004; 36:750–754.
17. Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, Kageyama R. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev. 2001; 15:2642–2647.
18. Takebayashi K, Sasai Y, Sakai Y, Watanabe T, Nakanishi S, Kageyama R. Structure, chromosomal locus, and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES-1. Negative autoregulation through the multiple N box elements. J Biol Chem. 1994; 269:5150–5156.
19. Kang SA, Seol JH, Kim J. The conserved WRPW motif of Hes6 mediates proteasomal degradation. Biochem Biophys Res Commun. 2005; 332:33–36.
20. Hay RT. SUMO: a history of modification. Mol Cell. 2005; 18:1–12.
21. Chiou HY, Liu SY, Lin CH, Lee EH. Hes-1 SUMOylation by protein inhibitor of activated STAT1 enhances the suppressing effect of Hes-1 on GADD45alpha expression to increase cell survival. J Biomed Sci. 2014; 21:53.
22. Denuc A, Marfany G. SUMO and ubiquitin paths converge. Biochem Soc Trans. 2010; 38(Pt 1):34–39.
23. Schimmel J, Larsen KM, Matic I, van Hagen M, Cox J, Mann M, et al. The ubiquitin-proteasome system is a key component of the SUMO-2/3 cycle. Mol Cell Proteomics. 2008; 7:2107–2122.
24. Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998; 2:233–239.
25. Lee J, Lee Y, Lee MJ, Park E, Kang SH, Chung CH, et al. Dual modification of BMAL1 by SUMO2/3 and ubiquitin promotes circadian activation of the CLOCK/BMAL1 complex. Mol Cell Biol. 2008; 28:6056–6065.
26. Cardone L, Hirayama J, Giordano F, Tamaru T, Palvimo JJ, Sassone-Corsi P. Circadian clock control by SUMOylation of BMAL1. Science. 2005; 309:1390–1394.
27. Kageyama R, Ohtsuka T, Kobayashi T. Roles of Hes genes in neural development. Dev Growth Differ. 2008; 50:Suppl 1. S97–S103.
28. Lee YJ, Han DH, Pak YK, Cho SH. Circadian regulation of low density lipoprotein receptor promoter activity by CLOCK/BMAL1, Hes1 and Hes6. Exp Mol Med. 2012; 44:642–652.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download