Abstract
Background
Metabolic syndrome (MetS), a cluster of multiple metabolic abnormalities, is one of the major public health challenges worldwide. The current study was conducted to evaluate the association between sugar-sweetened beverage (SSB) consumption and MetS and its components in Iranian adults.
Methods
This cross-sectional study was conducted among 5,852 men and women, aged 19 to 70 years, who participated in the fourth phase (2009 to 2011) of the Tehran Lipid and Glucose Study. Demographics, anthropometrics, biochemical measurements, and blood pressure (BP) were assessed and MetS was defined by National Cholesterol Education Program Adult Treatment Panel III definition. Frequency and quantity of SSB intakes including carbonated drinks and synthetic fruit juices were collected using a validated semiquantitative food frequency questionnaire.
Results
Mean age of participants (43%, men) was 40.6±12.9 years. Significant positive associations between SSBs and waist circumference, triglyceride level, systolic and diastolic BP in the third and fourth quartile of SSBs were observed, after adjustment for all potential confounding variables. The odds of MetS in the third and fourth quartiles compared to the first quartile category of SSBs was 1.21 (95% confidence interval [CI], 1.01 to 1.45) and 1.30 (95% CI, 1.06 to 1.58), respectively (P for trend=0.03). The odds of MetS, abdominal obesity, low high density lipoprotein cholesterol and elevated BP had increasing trends across increasing of SSB consumption (P for trend <0.05).
Metabolic syndrome (MetS), a cluster of multiple metabolic abnormalities including insulin resistance, central obesity, hyperglycemia, dyslipidemia, and hypertension, is one of the major public health challenges worldwide [1]. MetS increases the risk of chronic diseases including cardiovascular disease and diabetes. Moreover, of particular concern is unawareness of many people that they are suffering from this syndrome [2]. Different prevalences of MetS have been reported by studies, both Iranian and International. According to the Zabetian et al. [3] study, the prevalence of MetS was 32% among Iranian adults.
MetS is strongly related to life style factors including dietary patterns, smoking and physical activity [4]. Sugar-sweetened beverages (SSBs), liquid sources of added sugars in diet, could be one of these dietary determinants. One of the most commonly consumed added sugars is high-fructose corn syrup. It has been showed that the consumption of foods and beverages with a high content of fructose is associated with potential hormonal dysregulation, insulin resistance, dyslipidemia, and obesity [5]. Although associations between SSB consumption and MetS have been previously examined, results of these studies are inconsistent. A meta-analysis of prospective cohort studies has shown a positive association between SSB consumption and MetS [6]. In contrast, three reviews have shown that long-term sugar intakes have no adverse effects with respect to components of MetS and fructose consumption up to 140 g/day does not result in increase of triglycerides (TGs) in normal-weight, overweight, and obese people [789]. Furthermore, one cross-sectional study of Iranian adults found no association between SSB consumption and MetS [10]; however, there were concerns about accuracy of the assessment of SSB intakes, validity of biochemical variable measurements and the possibility of residual confounding in this study need to be considered in the interpretation of its results [10].
In the face of the epidemic of MetS and its strong association with cardiovascular complications, the current study was conducted to evaluate the association between SSB consumption and the MetS and its components in Iranian adults.
This study was conducted within the framework of the Tehran Lipid and Glucose Study (TLGS) between 2009 and 2011. Details of the TLGS have been reported elsewhere [1112]. Briefly, TLGS is a community-based prospective study being conducted to investigate and prevent noncommunicable diseases, in a representative sample of residents of district 13 of Tehran, the capital city of Iran. The first phase of the TLGS began in March 1999 and data collection is ongoing at 3-year intervals.
For the current study, 6,672 men and women aged 19 to 70 years, who participated in the fourth phase of TLGS (2009 to 2011) were recruited. We excluded participants if they lacked information on sex and anthropometric measurements (n=242), if they were under- or over reporters of dietary intakes (less than 800 kcal/day or more than 4,200 kcal/day, respectively; n=435), if they were on specific diets (n=130), or if they had reported cancer or stroke (n=13). After exclusions, the final analysis was conducted on data of 5,852 participants.
Informed written consents were obtained from all participants and the study protocol was approved by the research council of the Research Institute for Endocrine Sciences (RIES), Shahid Beheshti University of Medical Sciences. The study was conducted according to the Declaration of Helsinki and was approved by the Ethical Committee of the RIES, Shahid Beheshti University of Medical Sciences.
Dietary data were collected using a validated semiquantitative food frequency questionnaire (FFQ) with 168 food items, developed for the TLGS. Trained dietitians questioned participants regarding their intake frequency for each food item consumed during the past year on a daily, weekly, or monthly basis. The validity and reliability of the FFQ for food group intakes were assessed and were acceptable [13]. Because the Iranian Food Composition Table (FCT) is incomplete and has limited data on nutrient content of foods, we used the United States Department of Agriculture (USDA) FCT to analyze foods and beverages [14]; however, the Iranian FCT was used for some national foods and beverages, which are not listed in the USDA FCT [15]. The USDA database for added sugar was used to identify the added sugar contents of food items. Intake of added sugar was calculated in percent of energy per day [1416].
The FFQ included questions on the frequency of consumption and usual portion size of SSBs including carbonated drinks and synthetic fruit juices, both of which were combined to estimate the daily intake of SSBs. We calculated the total SSB consumption per day for each participant and for data analysis, portion sizes of consumed SSBs reported in household measures were converted to grams, and their intakes were categorized by using quartile cutoffs (<6.7, 6.7 to 21.8, 21.9 to 57.1, >57.1 g/day). Participants with dietary SSB intakes <6.7 g/day were considered as the reference group.
A structured questionnaire was used to collect data. Information on age (years), educational level (illiterate, primary education, and academic education), smoking behavior (yes/no), and physical activity level (metabolic equivalent hours per week [MET-hr/wk]) were also assessed. Participants who smoked daily or occasionally were considered current smokers, and those who had never smoked or those who had stopped smoking were considered nonsmokers. Physical activity level was assessed using the Persian translated Modifiable Activity Questionnaire (MAQ) [17]. High reliability and relatively moderate validity were reported for the Persian translated MAQ in Tehranian adults [18]. The frequency and time spent on light, moderate, hard, and very hard intensity activities according to the list of common activities of daily life over the past year were documented. We transformed the activity data into MET-hr/wk. Then the participants were categorized into low, moderate, and high activity groups [19].
Qualified examiners performed anthropometric measurements using a standardized process. Weight was measured to the nearest 100 g using digital scales, while the participants were minimally clothed, without shoes. Height was measured to the nearest 0.5 cm using a tape meter, in a standing position without shoes. Body mass index was calculated as weight (kg) divided by square of the height (m2). Waist circumference was measured to the nearest 0.1 cm, at the umbilical level over light clothing, using a tape meter. Body mass index ≥30 kg/m2 was classified as general obesity and waist circumference ≥95 cm for both sexes was classified as abdominal obesity [20]. Blood pressure (BP) was measured twice after a 15 minutes rest in the sitting position, on the right arm, using a standardized mercury sphygmomanometer. The mean of the two measurements was considered as the participant's BP.
Fasting blood samples were taken after 10 to 12 hours overnight fast. Fasting plasma glucose and TG were measured by the enzymatic colorimetric method and high density lipoprotein cholesterol (HDL-C) was measured using enzymatic photometric method. Analyses were performed using Parsazmun kits (Tehran, Iran) and a Selectra 2 autoanalyzer (Vital Scientific, Spankeren, Netherlands). Inter- and intra-assay coefficients of variation of all assays were <5% [11].
MetS was defined according to the National Cholesterol Education Program Adult Treatment Panel III definition, where a participant must meet ≥3 of the following metabolic abnormalities: (1) impaired fasting glucose (fasting plasma glucose ≥100 mg/dL or use of medication); (2) hypertriglyceridemia (TG ≥150 mg/dL or use of medication); (3) low HDL-C (HDL-C <40 mg/dL for men and <50 mg/dL for women); (4) elevated BP (BP ≥130/85 mm Hg or use of medication); (5) abdominal obesity (waist circumference ≥95 cm for both sexes) [2021].
All statistical analyses were conducted using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA), and P values <0.05 were considered significant. Participant characteristics were compared across quartiles of SSB intakes using the analysis of variance or the general linear model, adjusted for sex and age or the chi-square test. Dietary intakes of participants were compared across quartiles of SSB intakes, using one-way analysis of variance or Kruskal-Wallis test. The association between waist circumference, fasting blood sugar, TGs, HDL-C, systolic BP, and diastolic BP as continuous variables and quartiles of SSB intakes were determined using multivariable linear regression models with adjustment for age, sex, physical activity, smoking status, education status, and energy intake. Data are presented as β regression and 95% confidence interval (CI). The odds ratio (OR) of MetS and its components including abdominal obesity, impaired fasting glucose, hypertriglyceridemia, low HDL-C, and elevated BP in each quartile of SSB intake was determined using the multivariable logistic regression models. Logistic regression models included a dichotomous outcome (for example abdominal obesity [yes or no]) and quartiles of SSB intakes as the main predictor of interest, adjusted for age, sex, physical activity, smoking status, and education status. Because total energy intake could be in the casual pathway between SSB intakes and MetS risk, energy intake was adjusted in an additional model. Data are presented as OR with 95% CI. To assess the overall trends of OR across increasing quartiles of SSB intakes, the median SSB intakes of each quartile was used as a continuous variable in logistic regression models.
Mean age of participants was 40.6±12.9 years and 43% of the participants were men. Mean dietary intake of SSBs was 48.9±77.8 g/day (65.8±93.5 and 36.2±60.2 g/day, in men and women, respectively). The mean consumption of SSBs in the first, second, third, and fourth quartile categories was 2.6, 13.0, 36.1, and 144 g/day, respectively. Prevalence of the MetS in the overall population was 30.3% and among men and women was 38.0% and 24.4%, respectively. The distribution of MetS across quartiles of SSB intakes was not statistically significant (P=0.06).
Characteristics of the study participants across quartile categories of SSBs are shown in Table 1. Participants in the highest compared to the lowest quartile category of SSBs were more likely to be men (58.9% vs. 30.9%, P<0.001), more likely to be younger (36.2 years vs. 45.7 years, P<0.001), and more likely to be smokers (16.5% vs. 7.0%, P<0.001). Higher consumption of SSBs was also accompanied with lower physical activity (P=0.001) and higher education levels (P=0.02) in the study participants. Body mass index and waist circumference was significantly higher in the participant who were in the highest compared to the lowest quartile category of SSBs (P<0.001). Participants who consumed more SSBs had lower levels of fasting plasma glucose and HDL-C, and higher levels of TGs, systolic and diastolic BP (P<0.001).
Mean dietary intakes of participants across the quartile categories of SSB consumption are provided in Table 2. Participants in the upper quartile category had higher energy intakes (2,712 kcal/day vs. 2,207 kcal/day, P<0.001). There was no significant difference in dietary intake of carbohydrate and fat across quartiles of SSBs but dietary intakes of protein and total fiber decreased significantly across increasing trend of SSB consumption. Mean percent of added sugar from total energy in the highest, was over 2-fold that of the lowest quartile of SSB consumption (7.1% vs. 3.5% of total energy intake, P<0.01). Mean percent of added sugar from liquid sources in the first, second, third, and fourth quartile categories of SSB consumption was 2.6, 8.6, 16.9, and 34.2, respectively (P<0.001).
The association (β and 95% CI) of SSB consumption and the components of MetS in each quartile category of SSBs are presented in Table 3. Significant positive associations between SSBs and waist circumference, TG levels, systolic and diastolic BP in the third and fourth quartile of SSBs were observed after adjustment for all potential confounding variables including age, sex, physical activity, smoking status, education status, and energy intake. Higher consumption of SSBs was also associated to lower levels of fasting blood glucose and HDL-C.
The odds (95% CI) of MetS and its components in each quartile category of SSBs are shown in Table 4. After adjustment for all potential confounding variables, the odds of MetS in the third and fourth quartiles compared to the first quartile category of SSBs was 1.21 (95% CI, 1.01 to 1.45) and 1.30 (95% CI, 1.06 to 1.58), respectively (P for trend=0.03). Highest consumption of SSBs increased the odds of abdominal obesity by 35% (OR, 1.35; 95% CI, 1.12 to 1.61). The association between SSB consumption and impaired fasting glucose was not significant. Higher intake of SSBs was associated with the higher odds of hypertriglyceridemia only in the third quartile category of SSB consumption (OR, 1.23; 95% CI, 1.03 to 1.46). Participants who had higher consumption of SSBs (in the third and fourth quartile categories) also had higher odds of low HDL-C and elevated BP. The odds of MetS, abdominal obesity, low HDL-C and elevated BP had increasing trends across increasing categories of SSB consumption (P for trend <0.05).
In this cross-sectional study, higher intake of SSBs was associated with the higher odds of MetS in adults. We also observed that participants who consumed an average of 145 gram/day of SSBs had 35% higher odds of abdominal obesity, 24% higher odds of low HDL-C and 27% higher odds of elevated BP, compared with those in the lowest quartile of SSB consumption.
In the present study, mean dietary intake of SSBs was about 50 g/day. Balaghi et al. [22] in a study on Iranian university students reported that mean intake of soft drinks was 56 mL/day. In another study, Khosravi-Boroujeni et al. [10] have shown that mean intake of SSBs was lower than one serving per day in Iranian adults. These results indicated that SSBs had lower consumption in Iran compared to Western countries.
Current knowledge regarding the association of SSBs, MetS, and its components are controversial. Some studies support the positive associations between SSBs and MetS and its components [62324], whereas others do not confirm the associations [78910]. Different doses and forms of SSBs and different study designs and characteristics of participants could be responsible for these controversies in the various studies. Considering the fact that mean dietary intake of SSBs was lower in Iran compared to Western countries, we conducted this study and showed that higher intake of SSBs was associated with the higher odds of MetS in Iranian adults.
In the present study, the distribution of MetS across quartiles of SSB intakes was not statistically significant. It could be because of that subjects with high SSBs intake were more likely to be younger and age has higher impact on MetS compared to diet. However, after adjustment for all potential confounding variables, participants in the highest compared to the lowest quartile of SSB consumption had 30% higher odds of MetS. This observation is in agreement with cross-sectional findings from the Framingham Heart Study, which show that people who consumed one serving of SSBs daily were almost twice as likely to be metabolically unhealthy, compared to those who did not consume any SSB, independent of their weight status [23]. However, a previous cross-sectional study on Iranian adults failed to find any association between SSB consumption and MetS, it should be noted that Khosravi-Boroujeni et al. [10] collected no information on portion sizes of SSBs, and collected data only on SSB consumption frequency, raising concern about the accuracy of their assessment of SSB intakes. In this study, we estimated the total SSB consumption per day for each participant and different methods for assessing the SSB intake might provide a reason for conflicting results which have been observed in Iranian adults.
In this cross-sectional study, higher intake of SSBs was associated with the higher odds of abdominal obesity in adults, a finding confirming results of previous observational studies, which show regular consumption of SSBs to be associated with an increased risk of abdominal obesity [2526]. Studies have shown that liquid foods have low satiety effect and since SSBs are typically consumed in addition to usual food intake, they could increase the amount of energy intake [27]. In this study percentage of carbohydrate intake was not increased across quartiles of sugar sweetened beverage consumption. However, total energy and added sugar intakes increased across quartiles.
Palmer et al. [28] in a prospective study observed that regular consumption of SSBs is associated with incidence of type 2 diabetes. However, in this study the association between SSB consumption and impaired fasting glucose was not significant and higher consumption of SSBs was also associated to lower levels of fasting blood glucose, which could be because of the cross-sectional design of this study and a possibility of reverse causation. For example, patients with hyperglycemia may have reduced their SSB intake, thereby blunting the association between SSB consumption and hyperglycemia. Moreover, fasting blood glucose is a poor marker for average blood glucose levels compared to hemoglobin A1c (HbA1c). However, in the present study measurement of HbA1c is not affordable. Our result is in accordance with Khosravi-Boroujeni et al. [10] study which found no association between SSB consumption and hyperglycemia in Iranian adults.
In the present study, consumption of SSBs was also associated with dyslipidemia. Recently a meta-analysis reported that fructose consumption from industrialized foods such as SSBs is one of the causes of MetS in healthy adults [29]. High fructose corn syrup is now used as the sweeteners in SSBs; fructose can be converted to glycerol-3-phosphate without passing through the phosphofructokinase pathway and can hence be the cause of TG and fatty acid synthesis [30], furthermore, fructose decreases TG clearance by lipoprotein lipase [31]. SSB intake might also increase TG by stimulation of desaturase activities in the liver [32]. Therefore, increasing fructose consumption through manufactured products is a current issue of concern.
We showed that odds of elevated BP had a rising trend across increasing categories of SSB consumption. A recent systematic review reported compatible results, indicating that the consumption of SSBs is associated with higher BP, leading to increased incidence of hypertension [33]. The most known mechanism behind high SSB consumption and hypertension is an increase in uric acid production and the consequent lowering of nitric oxide in the body; nitric oxide is a vasodilator and its relative reduction can result in hypertension [3233]. Other mechanisms include decreased sodium excretion, increased sodium gut absorption and activation of the sympathetic nervous system [3334].
This study does have some limitations. Due to the cross-sectional nature of this study, no causality can be drawn between SSB consumption and MetS and potential for recall bias in the data is always there. The FFQ was used to estimate typical beverage consumption over the previous year, but like all dietary assessment methods, FFQs have their limitations and underreporting is probable, which could result in misclassification of exposure. Furthermore, although we attempted to control for major confounders in the present study, residual confounding cannot be ruled out. Cohorts with long-term follow up about the effect of SSB consumption on MetS and its components in Iranian population are recommended.
In conclusion, our study provides some evidence regarding the association of SSB consumption with higher odds of MetS in adults. Limiting intake of SSBs is one simple dietary behavioral change that could have a positive impact on MetS prevention and management.
Figures and Tables
Table 1
Characteristics of Participants according to Quartiles of Sugar-Sweetened Beverage Consumption (n=5,852)

Table 2
Dietary Intakes of Participants according to Quartiles of Sugar-Sweetened Beverage Consumption (n=5,852)

Table 3
The Association Between Sugar-sweetened Beverage Consumption and Metabolic Syndrome Components (n=5,852)

Table 4
OR and 95% CI for Metabolic Syndrome and Its Components across Quartile Categories of Sugar-Sweetened Beverage Consumption (n=5,852)
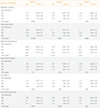
To assess the overall trends of OR across increasing quartiles of sugar-sweetened beverage (SSB) intakes, the median SSB intakes of each quartile was used as a continuous variable in logistic regression models.
OR, odds ratio; CI, confidence interval; HDL-C, high density lipoprotein cholesterol.
aModel 1, logistic regression model with adjustment for age and sex; bModel 2, additional adjustment for physical activity, smoking status and education status; cModel 3, additional adjustment for energy intake.
ACKNOWLEDGMENTS
The authors wish to acknowledge Ms. Niloofar Shiva for critical editing of English grammar and syntax of the manuscript. This work was funded by the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, I.R. Iran.
References
1. Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011; 9:48.
2. Schmidt AM. Insulin resistance and metabolic syndrome: mechanisms and consequences. Arterioscler Thromb Vasc Biol. 2012; 32:1753.
3. Zabetian A, Hadaegh F, Azizi F. Prevalence of metabolic syndrome in Iranian adult population, concordance between the IDF with the ATPIII and the WHO definitions. Diabetes Res Clin Pract. 2007; 77:251–257.
4. Bianchi C, Penno G, Daniele G, Benzi L, Del Prato S, Miccoli R. Optimizing management of metabolic syndrome to reduce risk: focus on life-style. Intern Emerg Med. 2008; 3:87–98.
5. Collino M. High dietary fructose intake: sweet or bitter life? World J Diabetes. 2011; 2:77–81.
6. Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010; 33:2477–2483.
7. Dolan LC, Potter SM, Burdock GA. Evidence-based review on the effect of normal dietary consumption of fructose on blood lipids and body weight of overweight and obese individuals. Crit Rev Food Sci Nutr. 2010; 50:889–918.
8. Dolan LC, Potter SM, Burdock GA. Evidence-based review on the effect of normal dietary consumption of fructose on development of hyperlipidemia and obesity in healthy, normal weight individuals. Crit Rev Food Sci Nutr. 2010; 50:53–84.
9. Ruxton CH, Gardner EJ, McNulty HM. Is sugar consumption detrimental to health? A review of the evidence 1995-2006. Crit Rev Food Sci Nutr. 2010; 50:1–19.
10. Khosravi-Boroujeni H, Sarrafzadegan N, Mohammadifard N, Alikhasi H, Sajjadi F, Asgari S, et al. Consumption of sugar-sweetened beverages in relation to the metabolic syndrome among Iranian adults. Obes Facts. 2012; 5:527–537.
11. Azizi F, Ghanbarian A, Momenan AA, Hadaegh F, Mirmiran P, Hedayati M, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials. 2009; 10:5.
12. Azizi F, Rahmani M, Emami H, Mirmiran P, Hajipour R, Madjid M, et al. Cardiovascular risk factors in an Iranian urban population: Tehran lipid and glucose study (phase 1). Soz Praventivmed. 2002; 47:408–426.
13. Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol. 2010; 20:150–158.
14. United States Department of Agriculture (USDA). Food composition table (FCT) [Internet]. Washington DC: USDA;2014. cited 2015 Jan 7. Available from: http://www.nal.usda.gov/fnic/foodcomp.
15. Azar M, Sarkisian E. Food composition table of Iran: National Nutrition and Food Research Institute. Tehran: Shahid Beheshti University Press;1980.
16. United States Department of Agriculture (USDA). USDA database for the added sugars content of selected foods [Internet]. Washington DC: USDA;2014. cited 2015 Jan 7. Available from: http://www.ars.usda.gov/nutrientdata/.
17. Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990; 13:401–411.
18. Momenan AA, Delshad M, Sarbazi N, Rezaei Ghaleh N, Ghanbarian A, Azizi F. Reliability and validity of the Modifiable Activity Questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med. 2012; 15:279–282.
19. IPAQ Research Committee. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ) [Internet]. International Physical Activity Questionnaire Research Committee;2005. cited 2015 Jan 8. Available from: http://www.ipaq.ki.se.
20. Azizi F, Hadaegh F, Khalili D, Esteghamati A, Hosseinpanah F, Delavari A, et al. Appropriate definition of metabolic syndrome among Iranian adults: report of the Iranian National Committee of Obesity. Arch Iran Med. 2010; 13:426–428.
21. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–2752.
22. Balaghi S, Faramarzi E, Mahdavi R, Ghaemmaghami J. Fluids intake and beverage consumption pattern among university students. Health Promot Perspect. 2011; 1:54–61.
23. Green AK, Jacques PF, Rogers G, Fox CS, Meigs JB, McKeown NM. Sugar-sweetened beverages and prevalence of the metabolically abnormal phenotype in the Framingham Heart Study. Obesity (Silver Spring). 2014; 22:E157–E163.
24. Barrio-Lopez MT, Martinez-Gonzalez MA, Fernandez-Montero A, Beunza JJ, Zazpe I, Bes-Rastrollo M. Prospective study of changes in sugar-sweetened beverage consumption and the incidence of the metabolic syndrome and its components: the SUN cohort. Br J Nutr. 2013; 110:1722–1731.
25. Odegaard AO, Choh AC, Czerwinski SA, Towne B, Demerath EW. Sugar-sweetened and diet beverages in relation to visceral adipose tissue. Obesity (Silver Spring). 2012; 20:689–691.
26. Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010; 121:1356–1364.
27. DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000; 24:794–800.
28. Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008; 168:1487–1492.
29. Kelishadi R, Mansourian M, Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition. 2014; 30:503–510.
30. Jurgens H, Haass W, Castaneda TR, Schurmann A, Koebnick C, Dombrowski F, et al. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res. 2005; 13:1146–1156.
31. Sievenpiper JL, Carleton AJ, Chatha S, Jiang HY, de Souza RJ, Beyene J, et al. Heterogeneous effects of fructose on blood lipids in individuals with type 2 diabetes: systematic review and meta-analysis of experimental trials in humans. Diabetes Care. 2009; 32:1930–1937.
32. Hostmark AT, Haug A. Does cheese intake blunt the association between soft drink intake and risk of the metabolic syndrome? Results from the cross-sectional Oslo Health Study. BMJ Open. 2012; 2:e001476.
33. Malik AH, Akram Y, Shetty S, Malik SS, Yanchou Njike V. Impact of sugar-sweetened beverages on blood pressure. Am J Cardiol. 2014; 113:1574–1580.
34. Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006; 290:F625–F631.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download