Abstract
The prevalence of diabetes is increasing globally, particularly in Asia. According to the 2013 Diabetes Atlas, an estimated 366 million people are affected by diabetes worldwide; 36% of those affected live in the Western Pacific region, with a significant proportion in East Asia. The reasons for this marked increase in the prevalence of diabetes can be extrapolated from several distinct features of the Asian region. First, the two most populated countries, China and India, are located in Asia. Second, Asians have experienced extremely rapid economic growth, including rapid changes in dietary patterns, during the past decades. As a result, Asians tend to have more visceral fat within the same body mass index range compared with Westerners. In addition, increased insulin resistance relative to reduced insulin secretory function is another important feature of Asian individuals with diabetes. Young age of disease onset is also a distinctive characteristic of these patients. Moreover, changing dietary patterns, such as increased consumption of white rice and processed red meat, contributes to the deteriorated lifestyle of this region. Recent studies suggest a distinctive responsiveness to novel anti-diabetic agents in Asia; however, further research and efforts to reverse the increasing prevalence of diabetes are needed worldwide.
Go to : 
The prevalence of diabetes is increasing worldwide, particularly in Asia. The Diabetes Atlas published in 2013 reported that 366 million individuals are affected by diabetes, and 36% of those affected live in the Western Pacific region, with a significant proportion in East Asia [1].
The alarming increase in the prevalence of diabetes in Asia can be explained in terms of several causes. The typical ethnic background of the Asian population involves a lower body mass index (BMI) with more visceral fat, a young age of diabetes onset, and significant historical changes during the past decades; all of these factors could contribute to a high prevalence of diabetes [2345678]. Another important contributor to the high prevalence of diabetes in Asia is the presence of the most populated countries, China and India. These two nations have the highest numbers of patients with diabetes in the world, they thus make a major contribution to the worldwide prevalence of diabetes [1].
This review will focus on those background and typical features of Asian populations that may contribute to the increasing prevalence of diabetes in Asia. In addition, pancreatic β-cell function in response to insulin sensitivity will be discussed, as it may be an important reason for the discrepancy in the prevalence of diabetes in Asian and Western countries. Finally, the responsiveness of Asians to recently developed treatment modalities will be discussed.
Go to : 
The Diabetes Atlas periodically publishes data on the prevalence of diabetes. The most recent Diabetes Atlas edition, from 2013, reported that, based on data on the current increasing prevalence, Asia and Africa will have the highest proportions of individuals with diabetes in 2030 [1]. For example, the prevalence of diabetes in China has increased dramatically, from approximately 1% in 1980 to 9.7% in the most recent estimate from a nationwide survey [9]. This increase is hypothesized to be due to increasing age, urbanization, positive family history, obesity, and hypertension [10]. A review highlighting data from the Korea National Health and Nutrition Examination Survey (KNHANES) indicated that the prevalence of diabetes in Koreans increased from 0.91% in 1971 to 9.9% in 2009 [4]. Additionally, they showed an increasing prevalence of prediabetes, indicating a large subgroup of individuals at high risk for diabetes, suggesting that prevention could be important to protect against a further increase in the prevalence of diabetes. A recent review regarding diabetes epidemiology in Asians [2] demonstrated that the increasing prevalence of diabetes is mirrored by undiagnosed diabetes and impaired glucose tolerance in various East Asian countries. The prevalence of diabetes in Asians is has increased, whereas that in Western countries has remained more stable during the last decades.
Go to : 
Data from the Diabetes Fact Sheet released by the Korean Diabetes Association in 2013 indicated the proportion of individuals with obesity, defined as BMI >25 kg/m2, reached 44.4%, indicating that almost half of the Korean individuals with diabetes are overweight [11]. The average BMI of Korean diabetic patients increased from 21.9 kg/m2 in 1989 to 1990 to 24.8 kg/m2 in 2010 to 2012 [1213]. A report based on the KNHANES from 2001 to 2013 found that the age-standardized prevalence of adult obesity increased from 29.2% to 31.8% [414]. The prevalence of diabetes during the same period increased in men. Although not all obese subjects develop diabetes, it is evident that obese individuals have a higher risk for the development of diabetes. As the prevalence of obesity is increasing in Asians, the prevalence of diabetes could increase further.
Go to : 
The onset of type 2 diabetes mellitus (T2DM) in younger age groups is likely to result in a major economic burden for countries in Asia due to premature ill health and the phenomenon of 'living long with chronic illness.' In developed countries, diabetes affects mainly those older than 65 years, as dietary patterns and energy intake have not changed dramatically in the past decades [15]. Although Asians are not accustomed to Western dietary patterns, most developing countries in Asia accept these as markers of economic development. The younger generations in Asian developing countries are born in relative wealth compared with their parents and, although they retain their parents' genetic background, they usually have access to an abundantly nutritional diet throughout their lives [16].
Given the genetic propensity for diabetes and the access to a diet that is abundantly nutritional, the age of diabetes onset is decreasing in these generations. According to the Centers for Disease Control and Prevention in the United States, the mean and median ages at which diabetes is initially detected among Americans aged 18 years or older were 53.7 and 53.8 years, respectively, in 1997 and 53.8 and 54.2 years, respectively, in 2011 [17]. According to analyses using KNHANES data [8], the prevalence of diabetes in Koreans subjects in their 40's was 5.9% in 2001, 5.7% in 2007, and 7.3% in 2013. Although the age of onset of diabetes in these groups did not evidently decrease, Korean men tend to develop diabetes approximately 3 years earlier than American men. The Hong Kong Diabetes Registry indicates that the mean age of diabetes onset is 52 years, which is relatively earlier than that in Caucasians [18]. Indeed, a multiethnic population-based cohort study in Canada found that the median age at diabetes diagnosis was 3 years younger in Chinese individuals compared with Caucasians (55 years vs. 58 years) [19]. Furthermore, the proportion of adults aged younger than 40 years with diabetes is higher in Asian countries compared with in the United States and Europe [1]. In a recent analysis from the Joint Asia Diabetes Association program [20], one in five adult diabetic individuals had young-onset diabetes, defined as a disease onset earlier than 40 years of age.
The increase in the prevalence and decrease in the age of onset of diabetes in young Asian generations may be explained by the increasing prevalence of obesity and abundant nutrition, including during the intrauterine period: that is, 'the thrifty genotype' hypothesis [21]. In addition, the behavioral patterns of younger children have been rapidly altered; fast food is readily available, and lifestyles are becoming increasingly sedentary due to Internet communication, computer games, television, academic pursuits, poor urban planning, and car travel [22]. A recent increase in the prevalence of gestational diabetes mellitus (GDM) in Asian countries may also contribute to the increase in diabetes in young Asians, as the offspring of mothers with GDM have increased adiposity at birth and increased risk of diabetes and obesity later in life [23]. Therefore, early exploration and education in high-risk or obese Asian youths are required for the prevention of diabetes.
Go to : 
Asian individuals generally have lower BMIs than individuals of other races, but they also tend to have higher visceral and body fat content. Multiethnic studies have reported that Asians of a given BMI have higher adiposity or visceral fat or waist circumference [24] and that the body fat of Asians is 3% to 5% higher than that of Caucasians with the same BMI [25]. In people of European origin, a BMI of 30 correlates with a body fat percentage of approximately 50% in men and 30% in women [26]. However, Asians of the same age and sex with the same BMI have a higher fat percentage; thus, they are at higher risk for diabetes, high blood pressure, and heart disease compared with individuals of other ethnic backgrounds [27]. This has been termed the 'Yudkin-Yajnik paradox' [28].
In addition, during the early stages of visceral fatness, the risk of dysglycemia is greater in some Asians than it is in Europeans of the same age [29]. These observations prompted the World Health Organization (WHO) to establish a specific Asia-Pacific guideline for the diagnosis of obesity, and various national organizations now recommend specific waist circumference values for their own countries [3031]. Greater visceral adiposity can lead to increased fatty acid influx to the liver, altered adipokine production, fatty liver, and hepatic insulin resistance [32]. Therefore, more intensive interventions should be used with Asian populations to prevent visceral fat accumulation.
Go to : 
Asians are known for dysfunctional pancreatic insulin secretory function. Many studies assessing glucose tolerance in healthy subjects have found slight insulin secretory defects in Asian individuals [3334]. A study performed in Koreans suggested that impaired insulin secretion was induced by insufficient pancreatic β-cell mass, functional defects within β-cells themselves, or a combination of both. They reported a linear correlation between β-cell mass and BMI in patients with T2DM, suggesting the possibility of reduced insulin secretory function in Asians due to lower BMI and, thus, smaller β-cell mass [35]. Furthermore, these studies suggested that maximal β-cell mass and regenerative capacity in response to insulin resistance is established at an early stage of life. In functional studies that analyzed pancreatic β-cell function via markers derived from oral glucose tolerance tests, Asian subjects with prediabetes displayed marked reductions in β-cell function with little change in insulin sensitivity [36]. From these results, it is clear that Asians have a higher risk of developing diabetes due to genetic defects affecting insulin secretory function and β-cell mass.
The exact mechanisms underlying reduced β-cell mass and its subsequent associations remain unknown. However, multiple genetic susceptibility factors were recently discovered. Several T2DM loci, including KCNQ1, were first discovered in Asians and replicated in Europeans [3738]. A recent meta-analysis of East Asian studies identified eight novel loci for T2DM; most of these variants were predicted to influence the risk of T2DM by affecting insulin secretion [37]. As these risk alleles are frequently found in East Asians, they may contribute to the reduced insulin secretory function seen in Asians; however, further research is required to investigate this association. In addition, Asians could benefit from treatment modalities that preserve or boost pancreatic islet functioning.
Go to : 
There have been tremendous changes in environmental, lifestyle, and dietary factors in Asia during the past few decades. Environmental changes have been similar to those of the Western world but have occurred on a different time scale [3]. Increased fast food and fat consumption, dramatic increases in the number of automobiles, and urbanization have contributed to steady increases in BMI and subsequent cardiometabolic risk factors.
Changes in dietary patterns may be the most important factor contributing to the increase in the prevalence of diabetes in Asia. These changes include rapid shifts from carbohydrates to fats, with increased intake of animal foods and sugars [39]. Additionally, increased white rice consumption is deleterious, as it has a high glycemic index [4041]; it is hypothesized that Asians have a higher glycemic response than Caucasians to processed white rice [42]. Dietary problems have also occurred due to the greater energy intake associated with the consumption of high-fat diets. Indeed, such diets were unknown when total caloric intake was low and simple carbohydrates were served as the main source of fuel. Therefore, individuals with a high risk of diabetes should be encouraged to eat healthy grains and plant proteins to prevent diabetes.
Go to : 
Asian individuals with diabetes are at increased risk of microvascular complications and albuminuria compared with Caucasians. In the WHO Multinational Study of Vascular Disease in Diabetes, the earliest study that compared vascular complications between ethnicities, Native Americans showed an increased incidence of retinopathy, renal complications, and lower extremity amputations as well as a higher incidence of ischemic heart disease compared with other cohorts [43]. In addition, Asian diabetic men had a higher incidence of albuminuria compared with European men.
These patterns of higher microvascular complications in Asians compared with Caucasians were replicated across multiple studies. In a cross-sectional study involving 32,308 patients with T2DM from 33 countries, Asian diabetic patients had the highest prevalence of microalbuminuria and/or macroalbuminuria [44]. In the Action in Diabetes and Vascular Disease (ADVANCE) study, patients with T2DM from Asia had a higher incidence of renal complications and ischemic stroke but a lower risk of coronary heart disease (CHD) and peripheral vascular disease than their counterparts in Eastern Europe [45]. These patterns were replicated in other studies [464748]. The exact mechanisms underlying these distinctive patterns remain unknown. Thus, further research is warranted to clarify the mechanisms underpinning the increased microvascular complications and ischemic stroke incidence as well as the lower incidence of CHD and peripheral vascular disease in Asian diabetic individuals.
Go to : 
Most treatment modalities for diabetes currently used in Western countries are available in Asia, and some of these are more popular in Asia than in the nation in which the drug was developed. In addition, most Asian countries have their own treatment algorithms, which differ from those of the American Diabetes Association, the European Association of the Study of Diabetes, and the International Diabetes Federation. However, these guidelines are affected primarily by the specific reimbursement system and economic status of each country. Nevertheless, it is important to discuss the differences in response to anti-diabetic medications.
An example of a preferred treatment option in Asians is the α-glucosidase inhibitor (AGI); however, it is not frequently prescribed in Western countries. In a recent analysis investigating the efficacy of an AGI, the acarbose in different ethnic groups, Southeast and East Asians had slightly better responses to acarbose compared with Europeans [49]. This class of drugs is more effective in individuals with high post-prandial glucose excursion due to a combination of dysfunctional insulin secretion and high carbohydrate intake [50].
Dipeptidyl peptidase 4 (DPP-4) inhibitors, novel anti-diabetic agents, act by inhibiting DPP, the enzyme that degrades the incretin hormones glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide, resulting in increased levels of their active forms [51]. DPP-4 inhibitors are known for their effects on post-prandial insulin secretion, prevention of islet apoptosis, and islet regeneration. A recent meta-analysis showed that DPP-4 inhibitors had a superior glucose-lowering efficacy in Asians than in other ethnic groups [52]. Indeed, Asian-dominant studies (≥50% Asian participants) resulted in hemoglobin A1c (HbA1c) changes of -0.92%, compared with changes of only -0.65% in non-Asian-dominant studies (<50% Asian participants), resulting in a net difference of -0.26%. Moreover, the most impressive finding of this study was a clear correlation between BMI and the HbA1c-lowering effect of DPP-4 inhibitors in Asian-dominant studies, a correlation that did not exist in non-Asian-dominant studies. Thus, DPP-4 inhibitors may have better glucose-lowering efficacy in Asians due to their lower BMIs, highlighting that these drugs may represent a better treatment option in Asians compared with Caucasians.
Go to : 
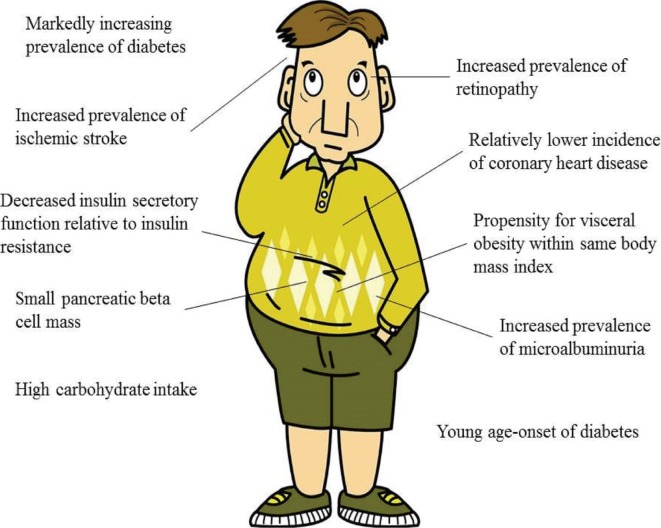
Asians are at higher risk for increased prevalence of diabetes. As the population of the Asia-Pacific region increases and accounts for a significant proportion of the world population, the worldwide prevalence of diabetes will continue to increase. Thus, an emphasis on interventions and the prevention of obesity and diabetes in Asians is essential to reverse this trend. The reasons for the increased prevalence of diabetes and the ethnic-specific characteristics of Asians are as follows: increasing obesity, a propensity for visceral obesity, impaired insulin secretion, reduced pancreatic β-cell mass, young age of diabetes onset, urbanization and modernization, increased microvascular complications and ischemic stroke, and better response to DPP-4 inhibitors (Fig. 1). However, further studies are warranted to clarify these exact mechanisms. Moreover, educational efforts and healthcare interventions are needed in Asia to reduce the global health risk of diabetes.
Go to : 
References
1. International Diabetes Federation. IDF diabetes atlas, 6th ed [Internet]. Brussels: International Diabetes Federation;c2014. cited 2015 Aug 18. Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf.
2. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013; 1281:64–91. PMID: 23551121.

3. Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006; 368:1681–1688. PMID: 17098087.

4. Kim DJ. The epidemiology of diabetes in Korea. Diabetes Metab J. 2011; 35:303–308. PMID: 21977448.

5. Weber MB, Oza-Frank R, Staimez LR, Ali MK, Narayan KM. Type 2 diabetes in Asians: prevalence, risk factors, and effectiveness of behavioral intervention at individual and population levels. Annu Rev Nutr. 2012; 32:417–439. PMID: 22524185.

6. Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci. 2013; 1281:51–63. PMID: 23317344.

7. Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010; 375:408–418. PMID: 19875164.

8. Ha KH, Kim DJ. Trends in the diabetes epidemic in Korea. Endocrinol Metab (Seoul). 2015; 30:142–146. PMID: 26194073.

9. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010; 362:1090–1101. PMID: 20335585.

10. Li H, Oldenburg B, Chamberlain C, O'Neil A, Xue B, Jolley D, et al. Diabetes prevalence and determinants in adults in China mainland from 2000 to 2010: a systematic review. Diabetes Res Clin Pract. 2012; 98:226–235. PMID: 22658670.

11. Korean Diabetes Association. Diabetes fact sheet in Korea 2013 [Internet]. Seoul: Korean Diabetes Association;c2011. cited 2015 Aug 18. Available from: http://www.diabetes.or.kr/temp/diabetes_factsheet_2013111.pdf.
12. Jeon JY, Kim DJ, Ko SH, Kwon HS, Lim S, Choi SH, et al. Current status of glycemic control of patients with diabetes in Korea: the fifth Korea National Health and Nutrition Examination Survey. Diabetes Metab J. 2014; 38:197–203. PMID: 25003073.

13. Ko KS, Oh TG, Kim CH, Park KS, Lee MK, Kim SY, et al. A clinical study on the complications of non-insulin-dependent diabetes mellitus in Korea. J Korean Diabetes Assoc. 1991; 15:257–262.
14. Korean Ministry of Health and Welfare. Korea health statistics 2012: Korea National Health and Nutrition Examination Survey. Seoul: Korean Ministry of Health and Welfare;2014.
15. Cockram CS. The epidemiology of diabetes mellitus in the Asia-Pacific region. Hong Kong Med J. 2000; 6:43–52. PMID: 10793402.
16. Popkin BM. The nutrition transition and its health implications in lower-income countries. Public Health Nutr. 1998; 1:5–21. PMID: 10555527.

17. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics. National Health and Nutrition Examination Survey, 2013 [Internet]. Hyattsville: Centers for Disease Control and Prevention;2014. updated 2014 Feb 3. cited 2015 Apr 27. Available from: http://www.cdc.gov.
18. Chan JC, So W, Ma RC, Tong PC, Wong R, Yang X. The complexity of vascular and non-vascular complications of diabetes: the Hong Kong Diabetes Registry. Curr Cardiovasc Risk Rep. 2011; 5:230–239. PMID: 21654912.

19. Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011; 34:1741–1748. PMID: 21680722.

20. Yeung RO, Zhang Y, Luk A, Yang W, Sobrepena L, Yoon KH, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014; 2:935–943. PMID: 25081582.

21. Sellayah D, Cagampang FR, Cox RD. On the evolutionary origins of obesity: a new hypothesis. Endocrinology. 2014; 155:1573–1588. PMID: 24605831.

22. Bar-Or O, Foreyt J, Bouchard C, Brownell KD, Dietz WH, Ravussin E, et al. Physical activity, genetic, and nutritional considerations in childhood weight management. Med Sci Sports Exerc. 1998; 30:2–10. PMID: 9475638.

23. Kim C. Gestational diabetes mellitus in korean women: similarities and differences from other racial/ethnic groups. Diabetes Metab J. 2014; 38:1–12. PMID: 24627822.

24. Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998; 22:1164–1171. PMID: 9877251.

25. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002; 3:141–146. PMID: 12164465.

26. Wang J, Thornton JC, Burastero S, Shen J, Tanenbaum S, Heymsfield SB, et al. Comparisons for body mass index and body fat percent among Puerto Ricans, blacks, whites and Asians living in the New York City area. Obes Res. 1996; 4:377–384. PMID: 8822762.

27. Araneta MR, Wingard DL, Barrett-Connor E. Type 2 diabetes and metabolic syndrome in Filipina-American women: a high-risk nonobese population. Diabetes Care. 2002; 25:494–499. PMID: 11874936.
29. Deurenberg-Yap M, Li T, Tan WL, van Staveren WA, Deurenberg P. Validation of a semiquantitative food frequency questionnaire for estimation of intakes of energy, fats and cholesterol among Singaporeans. Asia Pac J Clin Nutr. 2000; 9:282–288. PMID: 24394504.

30. World Health Organization, International Obesity Task Force. The Asian-Pacific perspective: redefining obesity and its treatment. Geneva: WHO Western Pacific Region;2000.
31. Kim MK, Lee WY, Kang JH, Kang JH, Kim BT, Kim SM, et al. 2014 Clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul). 2014; 29:405–409. PMID: 25559568.

32. Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010; 1801:209–214. PMID: 19948243.

33. Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004; 66(Suppl 1):S37–S43. PMID: 15563978.

34. Rattarasarn C, Soonthornpan S, Leelawattana R, Setasuban W. Decreased insulin secretion but not insulin sensitivity in normal glucose tolerant Thai subjects. Diabetes Care. 2006; 29:742–743. PMID: 16505544.

35. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003; 88:2300–2308. PMID: 12727989.
36. Taniguchi A, Fukushima M, Sakai M, Nagata I, Doi K, Nagasaka S, et al. Insulin secretion, insulin sensitivity, and glucose effectiveness in nonobese individuals with varying degrees of glucose tolerance. Diabetes Care. 2000; 23:127–128. PMID: 10857985.

37. Cho YS, Chen CH, Hu C, Long J, Ong RT, Sim X, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet. 2012; 44:67–72. PMID: 22158537.
38. Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet. 2008; 40:1092–1097. PMID: 18711367.

39. Popkin BM, Horton S, Kim S, Mahal A, Shuigao J. Trends in diet, nutritional status, and diet-related noncommunicable diseases in China and India: the economic costs of the nutrition transition. Nutr Rev. 2001; 59:379–390. PMID: 11766908.

40. Villegas R, Liu S, Gao YT, Yang G, Li H, Zheng W, et al. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med. 2007; 167:2310–2316. PMID: 18039989.

41. Nanri A, Mizoue T, Noda M, Takahashi Y, Kato M, Inoue M, et al. Rice intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr. 2010; 92:1468–1477. PMID: 20980490.

42. Henry CJ, Lightowler HJ, Newens K, Sudha V, Radhika G, Sathya RM, et al. Glycaemic index of common foods tested in the UK and India. Br J Nutr. 2008; 99:840–845. PMID: 17903341.

43. Lee ET, Lu M, Bennett PH, Keen H. Vascular disease in younger-onset diabetes: comparison of European, Asian and American Indian cohorts of the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001; 44(Suppl 2):S78–S81. PMID: 11587054.

44. Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG. DEMAND investigators. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006; 69:2057–2063. PMID: 16612330.

45. Clarke PM, Glasziou P, Patel A, Chalmers J, Woodward M, Harrap SB, et al. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med. 2010; 7:e1000236. PMID: 20186272.

46. Liu J, Hong Y, D'Agostino RB Sr, Wu Z, Wang W, Sun J, et al. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. JAMA. 2004; 291:2591–2599. PMID: 15173150.

47. Woodward M, Zhang X, Barzi F, Pan W, Ueshima H, Rodgers A, et al. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care. 2003; 26:360–366. PMID: 12547863.
48. Kim JH, Kim DJ, Jang HC, Choi SH. Epidemiology of micro-and macrovascular complications of type 2 diabetes in Korea. Diabetes Metab J. 2011; 35:571–577. PMID: 22247898.
49. Weng J, Soegondo S, Schnell O, Sheu WH, Grzeszczak W, Watada H, et al. Efficacy of acarbose in different geographical regions of the world: analysis of a real-life database. Diabetes Metab Res Rev. 2015; 31:155–167. PMID: 25044702.

50. Pan CY, Landen H. Post-marketing surveillance of acarbose treatment in patients with type 2 diabetes mellitus and subjects with impaired glucose tolerance in China. Clin Drug Investig. 2007; 27:397–405.

51. Gamble JM, Clarke A, Myers KJ, Agnew MD, Hatch K, Snow MM, et al. Incretin-based medications for type 2 diabetes: an overview of reviews. Diabetes Obes Metab. 2015; 17:649–658. PMID: 25772666.

52. Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013; 56:696–708. PMID: 23344728.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download