Abstract
Recent advances in molecular diagnostics have led to significant insights into the genetic basis of thyroid tumorigenesis. Among the mutations commonly seen in thyroid cancers, the vast majority are associated with the mitogen-activated protein kinase pathway. B-Raf proto-oncogene (BRAF) mutations are the most common mutations observed in papillary thyroid cancers (PTCs), followed by RET/PTC rearrangements and RAS mutations, while follicular thyroid cancers are more likely to harbor RAS mutations or PAX8/peroxisome proliferator-activated receptor γ (PPARγ) rearrangements. Beyond these more common mutations, alterations in the telomerase reverse transcriptase (TERT) promoter have recently been associated with clinicopathologic features, disease prognosis, and tumorigenesis in thyroid cancer. While the mutations underlying thyroid tumorigenesis are well known, the frequency of these mutations is strongly associated with geography, with clear differences reported between Asian and Western countries. Of particular interest is the prevalence of BRAF mutations, with Korean patients exhibiting the highest rate of BRAF-associated thyroid cancers in the world. Here, we review the prevalence of each of the most common mutations in Asian and Western countries, and identify the characteristics of well-differentiated thyroid cancer in Asians.
A number of genetic alterations have been shown to play a role in the development of follicular cell-derived thyroid cancer. These point mutations and translocations occur in genes of several important signaling pathways, particularly that of the mitogen-activated protein kinase (MAPK) pathway. The MAPK signaling pathway is a master regulator of numerous cellular processes including division, proliferation, differentiation, adhesion, migration, and apoptosis. B-Raf proto-oncogene (BRAF) mutations, RET/papillary thyroid cancer (PTC) rearrangements, and RAS mutations are the most common activators of the MAPK signaling pathway, with significant implications for thyroid tumorigenesis.
BRAF mutations are the most common mutations observed in PTCs, followed by RET/PTC rearrangements and RAS mutations, while follicular thyroid cancers (FTC) are more likely to harbor RAS mutations or PAX8/peroxisome proliferator-activated receptor γ (PPARγ) rearrangements. While all four of these mutations are common worldwide, the prevalence of each mutation type in thyroid cancer varies significantly, particularly between Asian and Western countries, with the prevalence of PTC significantly higher in Asian countries.
Beyond these more common mutations, alterations in the telomerase reverse transcriptase (TERT) promoter may be predictive of clinicopathologic features, as well as disease prognosis and tumorigenesis in thyroid cancer. Like other common thyroid cancer mutations, the frequency of TERT promoter mutations also appear to differ among countries, though the significance of this observation remains limited due to the small number of studies on this mutation having been conducted to date.
In this article, we review the prevalence of each of the most common mutations in Asian and Western countries, and identify the characteristics of well-differentiated thyroid cancer (DTC) in Asians.
BRAF, located in chromosome 7, is the most commonly mutated gene in thyroid cancers, resulting in potent activation of the MAPK pathway. The most common mutational hotspot in BRAF is T1799A in exon 15, conferring a glutamate to valine substitution at amino acid 600 (V600E) in the BRAF protein. BRAF V600E is the most common genetic alteration in PTC, exhibiting high prevalence in classic PTC and the tall cell variant, although it is generally rare in the follicular variant. Because BRAF mutations can be detected preoperatively in fine needle aspiration biopsy (FNAB) specimens, it is often used in the diagnosis of PTC, and may inform initial treatment strategies. Furthermore, this mutation has emerged as a promising prognostic factor for PTC [12], although the prognostic value of this mutation is still inconclusive [34].
The overall prevalence of BRAF mutations is ~45% (range, 27.3% to 87.1%) [56], with prevalence significantly higher in Asia, especially Korea, relative to Western countries (Table 1) [2356789101112131415161718192021222324]. Although the mechanisms underlying this difference in BRAF mutation frequencies are not well understood, a recent theory suggests that these differences may be associated with higher iodine intake in the Asian populations. Average iodine intakes were 138 to 353 µg/day in the United States [25], 45.3 µg/day in Germany [26], and 226 and 163 µg/day for women and men, respectively, in the United Kingdom [27]. Meanwhile, Japanese and Korean iodine intakes far exceed that of most other countries: 1,565 µg/day in Japan [28] and 479 µg/day in Korea [29]. Furthermore, high iodine intake has been shown to be significantly associated with the occurrence of BRAF mutation [7], though exceptions do exist, including lower BRAF mutation rates in Japan relative to Korea. One possible explanation for this discrepancy may be that of chronic thyroiditis, which is more prevalent in the Korean population. Incidence of Hashimoto's thyroiditis is strongly correlated with the development of PTC [30]. Because the prevalence of Hashimoto's thyroiditis is high in Korea, this positive correlation may provide an explanation for the high incidence of PTC in this country. However, as Hashimoto's thyroiditis is associated with genetic alterations other than BRAF mutations, such as rearrangements of RAS, ERK, and RET/PTC [31], the relationship between BRAF mutations in PTC and chronic thyroiditis requires further assessment.
While geographic differences in the incidence of BRAF mutations are well established, the prevalence of these mutations has changed over time. A recent publication from our laboratory revealed an increase in BRAF-associated thyroid cancers from 62.2% to 73.7% over the last two decades in Korea [8]. Similarly, in the United States, the overall prevalence of BRAF mutations remained stable for an extended period of time (~46%) but increased sharply from 50.0% to 76.9% in the classic papillary form of PTC over the last four decades [9]. More studies on the changes in the mutational rates and its clinical significance will be needed.
RAS mutations are the second most common genetic alteration in thyroid cancer. The RAS gene encodes a family of three isoforms: NRAS, HRAS, and KRAS. Thyroid neoplasms have been associated with mutations in all three isoforms of the RAS gene, although most studies have reported a predominance of NRAS61. RAS point mutations are commonly observed in FTC, as well as the follicular variant PTC. The frequency of RAS mutations in FTC ranges from 10.5% to 56.9% [3233], and is slightly more common in Asia (45.6%) than in Western countries (36.5% in the Americas, and 29.3% in Europe). In contrast, the frequency of RAS mutations in PTC is much lower in Asia (Table 2) [9323334353637383940414243444546474849505152]. This low frequency of RAS mutations has remained relatively stable over time, which is likely to be due to the lower prevalence of follicular variant PTC in this population. In contrast, a study from the United States reported an increase in the proportion of RAS mutation-positive from 2.7% between 1974 and 2000 to 24.9% in 2009, due in part to an increase in the percentage of patients presenting with the follicular variant histology [9].
RAS mutations have been reported in the full spectrum of thyroid neoplasms, limiting the clinical diagnostic value of these mutations. Because it is difficult to differentiate specific types of follicular lesions in thyroid FNAB samples, the diagnostic use of RAS mutations in FNAB specimens remains controversial. The prognostic value of RAS mutations is also unclear, although some evidence suggests that RAS-positive thyroid cancers may be at risk for tumor dedifferentiation, a less favorable prognosis, and metastatic behavior, particularly with regard to bone metastasis [3233].
Rearrangements of the RET proto-oncogene are commonly seen in PTC, and have been shown to play a role in disease pathogenesis. To date, 13 different types of RET/PTC rearrangements have been reported, though RET/PTC1 and RET/PTC3 account for more than 90% of all rearrangements. The relationship between radiation exposure and RET/PTC rearrangement has been established [5354], with RET/PTC rearrangements frequently observed in PTC patients who have received significant doses of external radiation, such as those affected by the Chernobyl nuclear accident. Elevated levels of childhood PTC are well documented in post-Chernobyl contaminated areas, accompanied by a high prevalence of RET/PTC rearrangements. Rapid proliferation of thyroid cells may account for the high sensitivity to radiation-induced RET/PTC rearrangements among children, although RET/PTC rearrangements also occur more frequently in children and young adults not exposed to radiation [55].
The prevalence of RET/PTC rearrangements in PTC varies widely in different populations (range, 0% to 86.8% [345356]), with significant variability in mutational frequency even within the same geographical regions (0% to 54.5% in Asia [345657], 2.4% to 72.0% in the Americas [958], and 8.1% to 42.9% in Europe [5960]). These discrepancies may be due to the small size of the studies; when this variability is taken into account, the prevalence of RET/PTC rearrangements in Asia is generally low (16.5%) (Table 3) [934425456575859606162636465666768697071].
This wide range of the prevalence rates seen in these studies may reflect not only the geographic variability but also the effect of different detection methods. A variety of methods have been used to identify RET/PTC rearrangements, including reverse transcription polymerase chain reaction methods, Southern blot analysis, and fluorescence in situ hybridization. Zhu et al. [72] demonstrated that different detection methods could result in significant variability in the detection of RET/PTC rearrangement.
PAX8/PPARγ rearrangements occur as a result of an intrachromosomal translocation between most of the coding sequence of PAX8 (2q13) and the entire coding exons of PPARγ1 (3p25). The fusion gene appears to be an oncogene, and results in production of a PAX8/PPARγ fusion protein (PPFP). The PAX8/PPARγ fusion gene is most commonly found in FTC, the follicular variant PTC, and benign follicular adenomas, though the prevalence of these rearrangements varies significantly among studies. The mean frequency in FTC is 5.6% in Asia, 43.8% in the Americas, and 27.4% in Europe (Table 4) [36384546737475767778798081828384]. The low frequency of PAX8/PPARγ rearrangements in Asia is particularly noteworthy, with one Japanese study failing to identify a single PAX8/PPARγ rearrangement in FTC [73].
No evidence exists linking PAX8/PPARγ rearrangements with clinical outcomes in FTC. Multiple studies have reported no correlation between PAX8/PPARγ rearrangements and clinical variables such as gender, age, tumor size, lymph node metastasis, recurrence, or mortality [747576].
Despite the lack of clinical associations, PPARγ remains an attractive therapeutic target in thyroid cancer. Although PPARγ agonists have shown promising results in both in vitro and in vivo studies [8586], the results of these studies have been inconclusive. Larger studies with long-term follow-up will be needed to clarify the efficacy and availability of PPARγ agonists in PPFP thyroid cancer.
Somatic mutations in the TERT promoter have been identified in many human malignancies including thyroid cancer. Mutations in the TERT promoter have been shown to increase telomerase activity, which protects the telomere repeats from erosion and plays a key role in cellular immortality and tumorigenesis [87]. TERT promoter mutations were mainly found in two hotspots, located -124 (chr5: 1,295,228C>T) and -146 bp (chr5: 1,295,250C>T) upstream of the gene transcription starting site. These mutations were recently shown to be more prevalent in aggressive thyroid cancers, and were associated with poor prognosis as well as high-risk clinicopathologic features [888990]. Therefore, TERT promoter mutation has received considerable attention as a novel prognostic biomarker. TERT promoter mutations have been shown to coexist with other tumorigenic alterations, such as BRAF or RAS mutations. Indeed, the coexistence of BRAF mutations and TERT promoter mutations has been identified as an indicator of the worst prognosis [8891].
The prevalence of TERT promoter mutation exhibits significant variability among countries ranging from 4.2% to 25.5% [8992] of PTC and 5.9% to 36.4% [9192] of FTC (Table 5) [88899091929394959697]. Among these, the Korean prevalence was noticeably lower than other countries. We analyzed 551 patients with DTC in our institution, with TERT promoter mutations identified in 4.5% of patients [92]. Among 222 DTCs treated at the Catholic University of Korea, the overall prevalence of TERT promoter mutations was 5.4% [93]. The relatively large proportion of small-size tumors in Korea may account for the low frequency of these mutations relative to other countries. TERT promoter mutation assays are difficult to use in routine prognostic testing of DTC, especially in areas where its prevalence is low. Therefore, further studies identifying an optimal subset of TERT promoter mutations may be warranted.
Recent advances in molecular diagnostics have led to significant insights into the genetic basis of thyroid tumorigenesis, including a number of genetic alterations involved in the development of follicular cell-derived cancers having been reported. The frequency of each of these mutations varies significantly among populations, with Asian residents exhibiting significantly different mutational profiles relative to Western countries. Korean populations often exhibit different mutation rates relative to other countries, with BRAF mutation rates higher than any other country, whereas RET/PTC and PAX8/PPARγ rearrangements, and TERT promoter mutations, are generally lower. Awareness of the role and prevalence of each mutation may be important for the design of future studies, and may hold promise as either a diagnostic tool or a therapeutic target.
References
1. Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010; 321:86–93. PMID: 19883729.

2. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013; 309:1493–1501. PMID: 23571588.

3. Ito Y, Yoshida H, Maruo R, Morita S, Takano T, Hirokawa M, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J. 2009; 56:89–97. PMID: 18840924.

4. Kim TY, Kim WB, Song JY, Rhee YS, Gong G, Cho YM, et al. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin Endocrinol (Oxf). 2005; 63:588–593. PMID: 16268813.
5. Goutas N, Vlachodimitropoulos D, Bouka M, Lazaris AC, Nasioulas G, Gazouli M. BRAF and K-RAS mutation in a Greek papillary and medullary thyroid carcinoma cohort. Anticancer Res. 2008; 28(1A):305–308. PMID: 18383861.
6. Kim SK, Song KH, Lim SD, Lim YC, Yoo YB, Kim JS, et al. Clinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent Hashimoto thyroiditis. Thyroid. 2009; 19:137–141. PMID: 19014278.

7. Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009; 94:1612–1617. PMID: 19190105.

8. Hong AR, Lim JA, Kim TH, Choi HS, Yoo WS, Min HS, et al. The frequency and clinical implications of the BRAF(V600E) mutation in papillary thyroid cancer patients in Korea over the past two decades. Endocrinol Metab (Seoul). 2014; 29:505–513. PMID: 25325273.
9. Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA Jr, Sigurdson AJ, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014; 99:E276–E285. PMID: 24248188.
10. Jo YS, Li S, Song JH, Kwon KH, Lee JC, Rha SY, et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab. 2006; 91:3667–3670. PMID: 16772349.

11. Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JH, et al. Role of BRAF V600E mutation as an indicator of the extent of thyroidectomy and lymph node dissection in conventional papillary thyroid carcinoma. Surgery. 2015; 6. 25. [Epub]. DOI: 10.1016/j.surg.2015.05.016.
12. Takahashi K, Eguchi H, Arihiro K, Ito R, Koyama K, Soda M, et al. The presence of BRAF point mutation in adult papillary thyroid carcinomas from atomic bomb survivors correlates with radiation dose. Mol Carcinog. 2007; 46:242–248. PMID: 17186541.
13. Ito Y, Yoshida H, Kihara M, Kobayashi K, Miya A, Miyauchi A. BRAF(V600E) mutation analysis in papillary thyroid carcinoma: is it useful for all patients? World J Surg. 2014; 38:679–687. PMID: 24052184.

14. Liu S, Zhang B, Zhao Y, Chen P, Ji M, Hou P, et al. Association of BRAFV600E mutation with clinicopathological features of papillary thyroid carcinoma: a study on a Chinese population. Int J Clin Exp Pathol. 2014; 7:6922–6928. PMID: 25400776.
15. Lu J, Gao J, Zhang J, Sun J, Wu H, Shi X, et al. Association between BRAF V600E mutation and regional lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2015; 8:793–799. PMID: 25755776.
16. Liu RT, Chen YJ, Chou FF, Li CL, Wu WL, Tsai PC, et al. No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clin Endocrinol (Oxf). 2005; 63:461–466. PMID: 16181240.

17. Kim J, Giuliano AE, Turner RR, Gaffney RE, Umetani N, Kitago M, et al. Lymphatic mapping establishes the role of BRAF gene mutation in papillary thyroid carcinoma. Ann Surg. 2006; 244:799–804. PMID: 17060774.

18. Oler G, Cerutti JM. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer. 2009; 115:972–980. PMID: 19152441.
19. Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, Stella M, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008; 15:191–205. PMID: 18310287.

20. Lupi C, Giannini R, Ugolini C, Proietti A, Berti P, Minuto M, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007; 92:4085–4090. PMID: 17785355.

21. Basolo F, Torregrossa L, Giannini R, Miccoli M, Lupi C, Sensi E, et al. Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab. 2010; 95:4197–4205. PMID: 20631031.
22. Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer. 2006; 13:257–269. PMID: 16601293.
23. Sykorova V, Dvorakova S, Ryska A, Vcelak J, Vaclavikova E, Laco J, et al. BRAFV600E mutation in the pathogenesis of a large series of papillary thyroid carcinoma in Czech Republic. J Endocrinol Invest. 2010; 33:318–324. PMID: 20009493.

24. Musholt TJ, Fottner C, Weber MM, Eichhorn W, Pohlenz J, Musholt PB, et al. Detection of papillary thyroid carcinoma by analysis of BRAF and RET/PTC1 mutations in fine-needle aspiration biopsies of thyroid nodules. World J Surg. 2010; 34:2595–2603. PMID: 20652698.

25. Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US Food and Drug Administration's Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol. 2008; 18:571–580. PMID: 18167505.

26. Scientific Committee on Food. Opinion of the scientific committee on food on the tolerable upper intake level of iodine. Brussels: European Commission, Health & Consumer Protection Directorate-General;2002.
27. Expert Group on Vitamins and Minerals. Safe upper levels for vitamins and minerals. London: Food Standards Agency;2003.
28. Ministry of Health, Labour and Welfare. Dietary reference intakes for Japanese. Tokyo: National Institute of Health and Nutrition;2010.
29. Kim JY, Moon SJ, Kim KR, Sohn CY, Oh JJ. Dietary iodine intake and urinary iodine excretion in normal Korean adults. Yonsei Med J. 1998; 39:355–362. PMID: 9752802.

30. Kim KW, Park YJ, Kim EH, Park SY, Park do J, Ahn SH, et al. Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto's thyroiditis. Head Neck. 2011; 33:691–695. PMID: 21484918.

31. Kang DY, Kim KH, Kim JM, Kim SH, Kim JY, Baik HW, et al. High prevalence of RET, RAS, and ERK expression in Hashimoto's thyroiditis and in papillary thyroid carcinoma in the Korean population. Thyroid. 2007; 17:1031–1038. PMID: 17900235.

32. Garcia-Rostan G, Zhao H, Camp RL, Pollan M, Herrero A, Pardo J, et al. ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003; 21:3226–3235. PMID: 12947056.

33. Fukahori M, Yoshida A, Hayashi H, Yoshihara M, Matsukuma S, Sakuma Y, et al. The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: new insights from a single center and a large patient cohort. Thyroid. 2012; 22:683–689. PMID: 22650231.

34. Park KY, Koh JM, Kim YI, Park HJ, Gong G, Hong SJ, et al. Prevalences of Gs alpha, ras, p53 mutations and ret/PTC rearrangement in differentiated thyroid tumours in a Korean population. Clin Endocrinol (Oxf). 1998; 49:317–323. PMID: 9861322.
35. Jang EK, Song DE, Sim SY, Kwon H, Choi YM, Jeon MJ, et al. NRAS codon 61 mutation is associated with distant metastasis in patients with follicular thyroid carcinoma. Thyroid. 2014; 24:1275–1281. PMID: 24820091.

36. Kim HJ, Jang HW, Sohn SY, Choi YL, Kim HJ, Oh YL, et al. Frequency of RAS mutations and PAX8/PPARgamma rearrangement in follicular thyroid tumors in Korea. Endocrinol Metab (Seoul). 2012; 27:45–53.
37. Park JY, Kim WY, Hwang TS, Lee SS, Kim H, Han HS, et al. BRAF and RAS mutations in follicular variants of papillary thyroid carcinoma. Endocr Pathol. 2013; 24:69–76. PMID: 23625203.

38. Jeong SH, Hong HS, Kwak JJ, Lee EH. Analysis of RAS mutation and PAX8/PPARgamma rearrangements in follicular-derived thyroid neoplasms in a Korean population: frequency and ultrasound findings. J Endocrinol Invest. 2015; 38:849–857. PMID: 25999051.
39. Lee SR, Jung CK, Kim TE, Bae JS, Jung SL, Choi YJ, et al. Molecular genotyping of follicular variant of papillary thyroid carcinoma correlates with diagnostic category of fine-needle aspiration cytology: values of RAS mutation testing. Thyroid. 2013; 23:1416–1422. PMID: 23590130.

40. Naito H, Pairojkul C, Kitahori Y, Yane K, Miyahara H, Konishi N, et al. Different ras gene mutational frequencies in thyroid papillary carcinomas in Japan and Thailand. Cancer Lett. 1998; 131:171–175. PMID: 9851250.

41. Kikuchi Y, Tsuji E, Yagi K, Matsusaka K, Tsuji S, Kurebayashi J, et al. Aberrantly methylated genes in human papillary thyroid cancer and their association with BRAF/RAS mutation. Front Genet. 2013; 4:271. PMID: 24367375.

42. Guo HQ, Zhao H, Zhang ZH, Zhu YL, Xiao T, Pan QJ. Impact of molecular testing in the diagnosis of thyroid fine needle aspiration cytology: data from mainland China. Dis Markers. 2014; 2014:912182. PMID: 24591770.

43. Liu RT, Hou CY, You HL, Huang CC, Hock L, Chou FF, et al. Selective occurrence of ras mutations in benign and malignant thyroid follicular neoplasms in Taiwan. Thyroid. 2004; 14:616–621. PMID: 15320975.

44. Namba H, Rubin SA, Fagin JA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol. 1990; 4:1474–1479. PMID: 2283998.

45. Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW 2nd, Tallini G, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003; 88:2318–2326. PMID: 12727991.
46. Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003; 120:71–77. PMID: 12866375.
47. Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010; 23:1191–1200. PMID: 20526288.

48. Lemoine NR, Mayall ES, Wyllie FS, Williams ED, Goyns M, Stringer B, et al. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene. 1989; 4:159–164. PMID: 2648253.
49. Esapa CT, Johnson SJ, Kendall-Taylor P, Lennard TW, Harris PE. Prevalence of Ras mutations in thyroid neoplasia. Clin Endocrinol (Oxf). 1999; 50:529–535. PMID: 10468914.
50. Basolo F, Pisaturo F, Pollina LE, Fontanini G, Elisei R, Molinaro E, et al. N-ras mutation in poorly differentiated thyroid carcinomas: correlation with bone metastases and inverse correlation to thyroglobulin expression. Thyroid. 2000; 10:19–23. PMID: 10691309.

51. Vasko V, Ferrand M, Di Cristofaro J, Carayon P, Henry JF, de Micco C. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J Clin Endocrinol Metab. 2003; 88:2745–2752. PMID: 12788883.

52. Di Cristofaro J, Marcy M, Vasko V, Sebag F, Fakhry N, Wynford-Thomas D, et al. Molecular genetic study comparing follicular variant versus classic papillary thyroid carcinomas: association of N-ras mutation in codon 61 with follicular variant. Hum Pathol. 2006; 37:824–830. PMID: 16784981.

53. Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997; 57:1690–1694. PMID: 9135009.
54. Elisei R, Romei C, Vorontsova T, Cosci B, Veremeychik V, Kuchinskaya E, et al. RET/PTC rearrangements in thyroid nodules: studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J Clin Endocrinol Metab. 2001; 86:3211–3216. PMID: 11443191.

55. Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000; 85:1170–1175. PMID: 10720057.
56. Namba H, Yamashita S, Pei HC, Ishikawa N, Villadolid MC, Tominaga T, et al. Lack of PTC gene (ret proto-oncogene rearrangement) in human thyroid tumors. Endocrinol Jpn. 1991; 38:627–632. PMID: 1823030.
57. Lee CH, Hsu LS, Chi CW, Chen GD, Yang AH, Chen JY. High frequency of rearrangement of the RET protooncogene (RET/PTC) in Chinese papillary thyroid carcinomas. J Clin Endocrinol Metab. 1998; 83:1629–1632. PMID: 9589668.

58. Rhoden KJ, Johnson C, Brandao G, Howe JG, Smith BR, Tallini G. Real-time quantitative RT-PCR identifies distinct c-RET, RET/PTC1 and RET/PTC3 expression patterns in papillary thyroid carcinoma. Lab Invest. 2004; 84:1557–1570. PMID: 15502856.

59. Mayr B, Potter E, Goretzki P, Ruschoff J, Dietmaier W, Hoang-Vu C, et al. Expression of Ret/PTC1, -2, -3, -delta3 and -4 in German papillary thyroid carcinoma. Br J Cancer. 1998; 77:903–906. PMID: 9528832.
60. Di Cristofaro J, Vasko V, Savchenko V, Cherenko S, Larin A, Ringel MD, et al. ret/PTC1 and ret/PTC3 in thyroid tumors from Chernobyl liquidators: comparison with sporadic tumors from Ukrainian and French patients. Endocr Relat Cancer. 2005; 12:173–183. PMID: 15788648.

61. Chung JH, Hahm JR, Min YK, Lee MS, Lee MK, Kim KW, et al. Detection of RET/PTC oncogene rearrangements in Korean papillary thyroid carcinomas. Thyroid. 1999; 9:1237–1243. PMID: 10646664.

62. Ishizaka Y, Ochiai M, Tahira T, Sugimura T, Nagao M. Activation of the ret-II oncogene without a sequence encoding a transmembrane domain and transforming activity of two ret-II oncogene products differing in carboxy-termini due to alternative splicing. Oncogene. 1989; 4:789–794. PMID: 2734021.
63. Wajjwalku W, Nakamura S, Hasegawa Y, Miyazaki K, Satoh Y, Funahashi H, et al. Low frequency of rearrangements of the ret and trk proto-oncogenes in Japanese thyroid papillary carcinomas. Jpn J Cancer Res. 1992; 83:671–675. PMID: 1381340.
64. Motomura T, Nikiforov YE, Namba H, Ashizawa K, Nagataki S, Yamashita S, et al. ret rearrangements in Japanese pediatric and adult papillary thyroid cancers. Thyroid. 1998; 8:485–489. PMID: 9669285.

65. Nibu K, Otsuki N, Nakao K, Sugasawa M, Rothstein JL. RET/PTC fusion gene rearrangements in Japanese thyroid carcinomas. Eur Arch Otorhinolaryngol. 2005; 262:368–373. PMID: 15368067.

66. Lam AK, Montone KT, Nolan KA, Livolsi VA. Ret oncogene activation in papillary thyroid carcinoma: prevalence and implication on the histological parameters. Hum Pathol. 1998; 29:565–568. PMID: 9635675.

67. Tallini G, Santoro M, Helie M, Carlomagno F, Salvatore G, Chiappetta G, et al. RET/PTC oncogene activation defines a subset of papillary thyroid carcinomas lacking evidence of progression to poorly differentiated or undifferentiated tumor phenotypes. Clin Cancer Res. 1998; 4:287–294. PMID: 9516913.
68. Sugg SL, Ezzat S, Zheng L, Freeman JL, Rosen IB, Asa SL. Oncogene profile of papillary thyroid carcinoma. Surgery. 1999; 125:46–52. PMID: 9889797.

69. Musholt TJ, Musholt PB, Khaladj N, Schulz D, Scheumann GF, Klempnauer J. Prognostic significance of RET and NTRK1 rearrangements in sporadic papillary thyroid carcinoma. Surgery. 2000; 128:984–993. PMID: 11114633.

70. Cinti R, Yin L, Ilc K, Berger N, Basolo F, Cuccato S, et al. RET rearrangements in papillary thyroid carcinomas and adenomas detected by interphase FISH. Cytogenet Cell Genet. 2000; 88:56–61. PMID: 10773666.

71. Puxeddu E, Moretti S, Giannico A, Martinelli M, Marino C, Avenia N, et al. Ret/PTC activation does not influence clinical and pathological features of adult papillary thyroid carcinomas. Eur J Endocrinol. 2003; 148:505–513. PMID: 12720532.

72. Zhu Z, Ciampi R, Nikiforova MN, Gandhi M, Nikiforov YE. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab. 2006; 91:3603–3610. PMID: 16772343.
73. Hibi Y, Nagaya T, Kambe F, Imai T, Funahashi H, Nakao A, et al. Is thyroid follicular cancer in Japanese caused by a specific t(2; 3)(q13; p25) translocation generating Pax8-PPAR gamma fusion mRNA. Endocr J. 2004; 51:361–366. PMID: 15256783.
74. Nikiforova MN, Biddinger PW, Caudill CM, Kroll TG, Nikiforov YE. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol. 2002; 26:1016–1023. PMID: 12170088.
75. Sahin M, Allard BL, Yates M, Powell JG, Wang XL, Hay ID, et al. PPARgamma staining as a surrogate for PAX8/PPARgamma fusion oncogene expression in follicular neoplasms: clinicopathological correlation and histopathological diagnostic value. J Clin Endocrinol Metab. 2005; 90:463–468. PMID: 15483076.
76. Boos LA, Dettmer M, Schmitt A, Rudolph T, Steinert H, Moch H, et al. Diagnostic and prognostic implications of the PAX8-PPARgamma translocation in thyroid carcinomas-a TMA-based study of 226 cases. Histopathology. 2013; 63:234–241. PMID: 23738683.
77. French CA, Alexander EK, Cibas ES, Nose V, Laguette J, Faquin W, et al. Genetic and biological subgroups of low-stage follicular thyroid cancer. Am J Pathol. 2003; 162:1053–1060. PMID: 12651598.

78. Giordano TJ, Au AY, Kuick R, Thomas DG, Rhodes DR, Wilhelm KG Jr, et al. Delineation, functional validation, and bioinformatic evaluation of gene expression in thyroid follicular carcinomas with the PAX8-PPARG translocation. Clin Cancer Res. 2006; 12(7 Pt 1):1983–1993. PMID: 16609007.

79. Nakabashi CC, Guimaraes GS, Michaluart P Jr, Ward LS, Cerutti JM, Maciel RM. The expression of PAX8-PPARgamma rearrangements is not specific to follicular thyroid carcinoma. Clin Endocrinol (Oxf). 2004; 61:280–282. PMID: 15272927.

80. Dwight T, Thoppe SR, Foukakis T, Lui WO, Wallin G, Hoog A, et al. Involvement of the PAX8/peroxisome proliferator-activated receptor gamma rearrangement in follicular thyroid tumors. J Clin Endocrinol Metab. 2003; 88:4440–4445. PMID: 12970322.
81. Lacroix L, Lazar V, Michiels S, Ripoche H, Dessen P, Talbot M, et al. Follicular thyroid tumors with the PAX8-PPARgamma1 rearrangement display characteristic genetic alterations. Am J Pathol. 2005; 167:223–231. PMID: 15972966.
82. Di Cristofaro J, Silvy M, Lanteaume A, Marcy M, Carayon P, De Micco C. Expression of tpo mRNA in thyroid tumors: quantitative PCR analysis and correlation with alterations of ret, Braf, ras and pax8 genes. Endocr Relat Cancer. 2006; 13:485–495. PMID: 16728576.
83. Castro P, Rebocho AP, Soares RJ, Magalhaes J, Roque L, Trovisco V, et al. PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2006; 91:213–220. PMID: 16219715.
84. Sahpaz A, Onal B, Yesilyurt A, Han U, Delibasi T. BRAF (V600E) mutation, RET/PTC1 and PAX8-PPAR gamma rearrangements in follicular epithelium derived thyroid lesions: institutional experience and literature review. Balkan Med J. 2015; 32:156–166. PMID: 26167339.
85. Park JW, Zarnegar R, Kanauchi H, Wong MG, Hyun WC, Ginzinger DG, et al. Troglitazone, the peroxisome proliferator-activated receptor-gamma agonist, induces antiproliferation and redifferentiation in human thyroid cancer cell lines. Thyroid. 2005; 15:222–231. PMID: 15785241.
86. Aiello A, Pandini G, Frasca F, Conte E, Murabito A, Sacco A, et al. Peroxisomal proliferator-activated receptor-gamma agonists induce partial reversion of epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Endocrinology. 2006; 147:4463–4475. PMID: 16777971.
87. Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994; 266:2011–2015. PMID: 7605428.

88. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014; 32:2718–2726. PMID: 25024077.

89. Liu T, Wang N, Cao J, Sofiadis A, Dinets A, Zedenius J, et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene. 2014; 33:4978–4984. PMID: 24141777.

90. Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2014; 99:E754–E765. PMID: 24476079.

91. Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab. 2014; 99:E1130–E1136. PMID: 24617711.
92. Song YS, Lim JA, Kim YA, Hwangbo Y, Kim KW, Min HS, et al. The effects of TERT promoter mutation and coexisting mutations on poor outcome in thyroid cancer. Paper presented at: 2015 Seoul International Congress of Endocrinology and Metabolism. 2015 Apr 30-May 3; Seoul, Korea.
93. Jung CK, Bae JS, Kim YR, Jeon SR, Kim SH, Kim TJ, et al. The role of TERT promoter mutations and ALK rearrangement in thyroid cancer patients with a high prevalence of the BRAF V600E mutation. Paper presented at: Annual Autumn Meeting of the Korean Thyroid Association. 2015 Aug 28-29; Daegu, Korea.
94. Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013; 20:603–610. PMID: 23766237.

95. Wang N, Liu T, Sofiadis A, Juhlin CC, Zedenius J, Hoog A, et al. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer. 2014; 120:2965–2979. PMID: 24898513.
96. Muzza M, Colombo C, Rossi S, Tosi D, Cirello V, Perrino M, et al. Telomerase in differentiated thyroid cancer: promoter mutations, expression and localization. Mol Cell Endocrinol. 2015; 399:288–295. PMID: 25448848.

97. Gandolfi G, Ragazzi M, Frasoldati A, Piana S, Ciarrocchi A, Sancisi V. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. Eur J Endocrinol. 2015; 172:403–413. PMID: 25583906.

Table 1
The Prevalence of BRAF Mutations in Papillary Thyroid Cancers
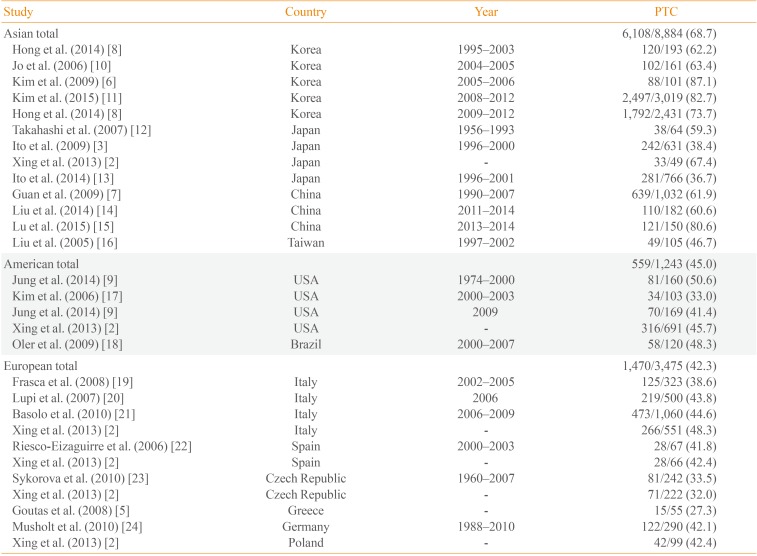
| Study | Country | Year | PTC |
|---|---|---|---|
| Asian total | 6,108/8,884 (68.7) | ||
| Hong et al. (2014) [8] | Korea | 1995-2003 | 120/193 (62.2) |
| Jo et al. (2006) [10] | Korea | 2004-2005 | 102/161 (63.4) |
| Kim et al. (2009) [6] | Korea | 2005-2006 | 88/101 (87.1) |
| Kim et al. (2015) [11] | Korea | 2008-2012 | 2,497/3,019 (82.7) |
| Hong et al. (2014) [8] | Korea | 2009-2012 | 1,792/2,431 (73.7) |
| Takahashi et al. (2007) [12] | Japan | 1956-1993 | 38/64 (59.3) |
| Ito et al. (2009) [3] | Japan | 1996-2000 | 242/631 (38.4) |
| Xing et al. (2013) [2] | Japan | - | 33/49 (67.4) |
| Ito et al. (2014) [13] | Japan | 1996-2001 | 281/766 (36.7) |
| Guan et al. (2009) [7] | China | 1990-2007 | 639/1,032 (61.9) |
| Liu et al. (2014) [14] | China | 2011-2014 | 110/182 (60.6) |
| Lu et al. (2015) [15] | China | 2013-2014 | 121/150 (80.6) |
| Liu et al. (2005) [16] | Taiwan | 1997-2002 | 49/105 (46.7) |
| American total | 559/1,243 (45.0) | ||
| Jung et al. (2014) [9] | USA | 1974-2000 | 81/160 (50.6) |
| Kim et al. (2006) [17] | USA | 2000-2003 | 34/103 (33.0) |
| Jung et al. (2014) [9] | USA | 2009 | 70/169 (41.4) |
| Xing et al. (2013) [2] | USA | - | 316/691 (45.7) |
| Oler et al. (2009) [18] | Brazil | 2000-2007 | 58/120 (48.3) |
| European total | 1,470/3,475 (42.3) | ||
| Frasca et al. (2008) [19] | Italy | 2002-2005 | 125/323 (38.6) |
| Lupi et al. (2007) [20] | Italy | 2006 | 219/500 (43.8) |
| Basolo et al. (2010) [21] | Italy | 2006-2009 | 473/1,060 (44.6) |
| Xing et al. (2013) [2] | Italy | - | 266/551 (48.3) |
| Riesco-Eizaguirre et al. (2006) [22] | Spain | 2000-2003 | 28/67 (41.8) |
| Xing et al. (2013) [2] | Spain | - | 28/66 (42.4) |
| Sykorova et al. (2010) [23] | Czech Republic | 1960-2007 | 81/242 (33.5) |
| Xing et al. (2013) [2] | Czech Republic | - | 71/222 (32.0) |
| Goutas et al. (2008) [5] | Greece | - | 15/55 (27.3) |
| Musholt et al. (2010) [24] | Germany | 1988-2010 | 122/290 (42.1) |
| Xing et al. (2013) [2] | Poland | - | 42/99 (42.4) |
Table 2
The Prevalence of RAS Mutations in Well-Differentiated Thyroid Cancers
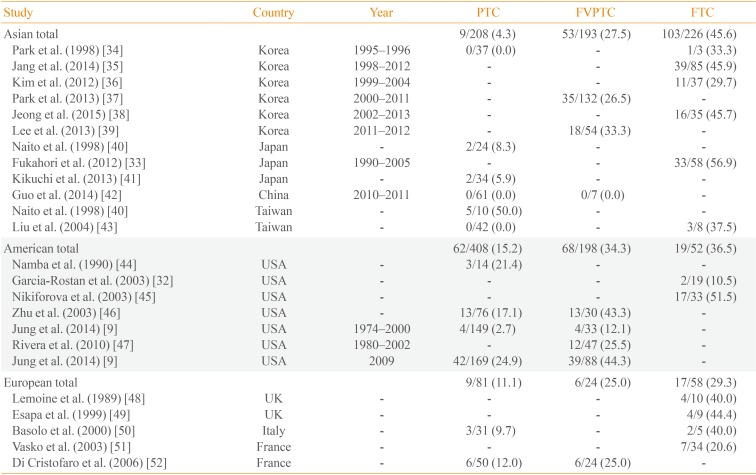
| Study | Country | Year | PTC | FVPTC | FTC |
|---|---|---|---|---|---|
| Asian total | 9/208 (4.3) | 53/193 (27.5) | 103/226 (45.6) | ||
| Park et al. (1998) [34] | Korea | 1995-1996 | 0/37 (0.0) | - | 1/3 (33.3) |
| Jang et al. (2014) [35] | Korea | 1998-2012 | - | - | 39/85 (45.9) |
| Kim et al. (2012) [36] | Korea | 1999-2004 | - | - | 11/37 (29.7) |
| Park et al. (2013) [37] | Korea | 2000-2011 | - | 35/132 (26.5) | - |
| Jeong et al. (2015) [38] | Korea | 2002-2013 | - | - | 16/35 (45.7) |
| Lee et al. (2013) [39] | Korea | 2011-2012 | - | 18/54 (33.3) | - |
| Naito et al. (1998) [40] | Japan | - | 2/24 (8.3) | - | - |
| Fukahori et al. (2012) [33] | Japan | 1990-2005 | - | - | 33/58 (56.9) |
| Kikuchi et al. (2013) [41] | Japan | - | 2/34 (5.9) | - | - |
| Guo et al. (2014) [42] | China | 2010-2011 | 0/61 (0.0) | 0/7 (0.0) | - |
| Naito et al. (1998) [40] | Taiwan | - | 5/10 (50.0) | - | - |
| Liu et al. (2004) [43] | Taiwan | - | 0/42 (0.0) | - | 3/8 (37.5) |
| American total | 62/408 (15.2) | 68/198 (34.3) | 19/52 (36.5) | ||
| Namba et al. (1990) [44] | USA | - | 3/14 (21.4) | - | - |
| Garcia-Rostan et al. (2003) [32] | USA | - | - | - | 2/19 (10.5) |
| Nikiforova et al. (2003) [45] | USA | - | - | - | 17/33 (51.5) |
| Zhu et al. (2003) [46] | USA | - | 13/76 (17.1) | 13/30 (43.3) | - |
| Jung et al. (2014) [9] | USA | 1974-2000 | 4/149 (2.7) | 4/33 (12.1) | - |
| Rivera et al. (2010) [47] | USA | 1980-2002 | - | 12/47 (25.5) | - |
| Jung et al. (2014) [9] | USA | 2009 | 42/169 (24.9) | 39/88 (44.3) | - |
| European total | 9/81 (11.1) | 6/24 (25.0) | 17/58 (29.3) | ||
| Lemoine et al. (1989) [48] | UK | - | - | - | 4/10 (40.0) |
| Esapa et al. (1999) [49] | UK | - | - | - | 4/9 (44.4) |
| Basolo et al. (2000) [50] | Italy | - | 3/31 (9.7) | - | 2/5 (40.0) |
| Vasko et al. (2003) [51] | France | - | - | - | 7/34 (20.6) |
| Di Cristofaro et al. (2006) [52] | France | - | 6/50 (12.0) | 6/24 (25.0) | - |
Table 3
The Prevalence of RET/PTC Rearrangements in Papillary Thyroid Cancers
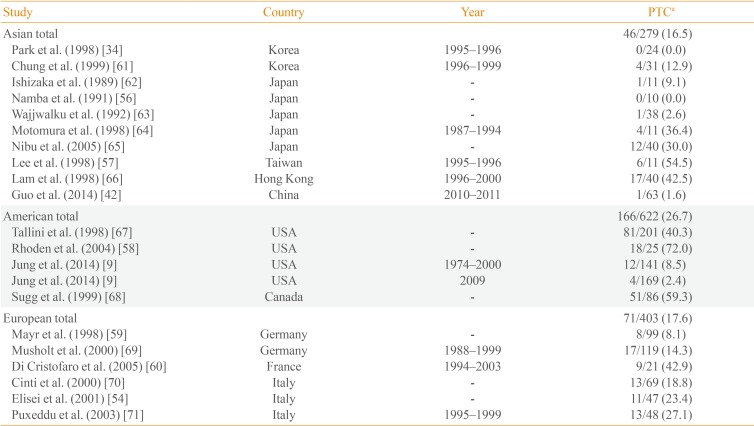
| Study | Country | Year | PTCa |
|---|---|---|---|
| Asian total | 46/279 (16.5) | ||
| Park et al. (1998) [34] | Korea | 1995-1996 | 0/24 (0.0) |
| Chung et al. (1999) [61] | Korea | 1996-1999 | 4/31 (12.9) |
| Ishizaka et al. (1989) [62] | Japan | - | 1/11 (9.1) |
| Namba et al. (1991) [56] | Japan | - | 0/10 (0.0) |
| Wajjwalku et al. (1992) [63] | Japan | - | 1/38 (2.6) |
| Motomura et al. (1998) [64] | Japan | 1987-1994 | 4/11 (36.4) |
| Nibu et al. (2005) [65] | Japan | - | 12/40 (30.0) |
| Lee et al. (1998) [57] | Taiwan | 1995-1996 | 6/11 (54.5) |
| Lam et al. (1998) [66] | Hong Kong | 1996-2000 | 17/40 (42.5) |
| Guo et al. (2014) [42] | China | 2010-2011 | 1/63 (1.6) |
| American total | 166/622 (26.7) | ||
| Tallini et al. (1998) [67] | USA | - | 81/201 (40.3) |
| Rhoden et al. (2004) [58] | USA | - | 18/25 (72.0) |
| Jung et al. (2014) [9] | USA | 1974-2000 | 12/141 (8.5) |
| Jung et al. (2014) [9] | USA | 2009 | 4/169 (2.4) |
| Sugg et al. (1999) [68] | Canada | - | 51/86 (59.3) |
| European total | 71/403 (17.6) | ||
| Mayr et al. (1998) [59] | Germany | - | 8/99 (8.1) |
| Musholt et al. (2000) [69] | Germany | 1988-1999 | 17/119 (14.3) |
| Di Cristofaro et al. (2005) [60] | France | 1994-2003 | 9/21 (42.9) |
| Cinti et al. (2000) [70] | Italy | - | 13/69 (18.8) |
| Elisei et al. (2001) [54] | Italy | - | 11/47 (23.4) |
| Puxeddu et al. (2003) [71] | Italy | 1995-1999 | 13/48 (27.1) |
Table 4
The Prevalence of PAX8/PPARγ Rearrangements in Well-Differentiated Thyroid Cancers
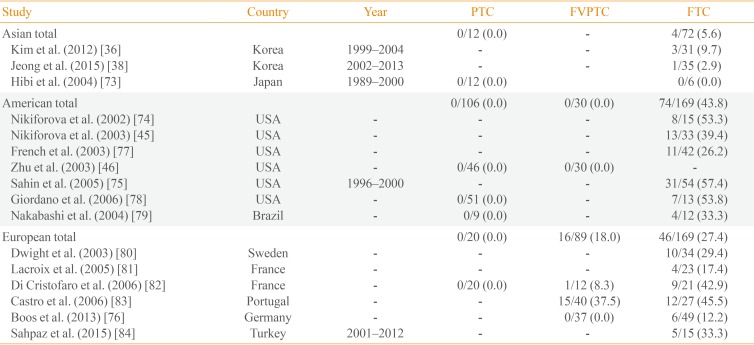
| Study | Country | Year | PTC | FVPTC | FTC |
|---|---|---|---|---|---|
| Asian total | 0/12 (0.0) | - | 4/72 (5.6) | ||
| Kim et al. (2012) [36] | Korea | 1999-2004 | - | - | 3/31 (9.7) |
| Jeong et al. (2015) [38] | Korea | 2002-2013 | - | - | 1/35 (2.9) |
| Hibi et al. (2004) [73] | Japan | 1989-2000 | 0/12 (0.0) | 0/6 (0.0) | |
| American total | 0/106 (0.0) | 0/30 (0.0) | 74/169 (43.8) | ||
| Nikiforova et al. (2002) [74] | USA | - | - | - | 8/15 (53.3) |
| Nikiforova et al. (2003) [45] | USA | - | - | - | 13/33 (39.4) |
| French et al. (2003) [77] | USA | - | - | - | 11/42 (26.2) |
| Zhu et al. (2003) [46] | USA | - | 0/46 (0.0) | 0/30 (0.0) | - |
| Sahin et al. (2005) [75] | USA | 1996-2000 | - | - | 31/54 (57.4) |
| Giordano et al. (2006) [78] | USA | - | 0/51 (0.0) | - | 7/13 (53.8) |
| Nakabashi et al. (2004) [79] | Brazil | - | 0/9 (0.0) | - | 4/12 (33.3) |
| European total | 0/20 (0.0) | 16/89 (18.0) | 46/169 (27.4) | ||
| Dwight et al. (2003) [80] | Sweden | - | - | - | 10/34 (29.4) |
| Lacroix et al. (2005) [81] | France | - | - | - | 4/23 (17.4) |
| Di Cristofaro et al. (2006) [82] | France | - | 0/20 (0.0) | 1/12 (8.3) | 9/21 (42.9) |
| Castro et al. (2006) [83] | Portugal | - | - | 15/40 (37.5) | 12/27 (45.5) |
| Boos et al. (2013) [76] | Germany | - | - | 0/37 (0.0) | 6/49 (12.2) |
| Sahpaz et al. (2015) [84] | Turkey | 2001-2012 | - | - | 5/15 (33.3) |
Table 5
The Prevalence of TERT Promoter Mutations in Well-Differentiated Thyroid Cancers
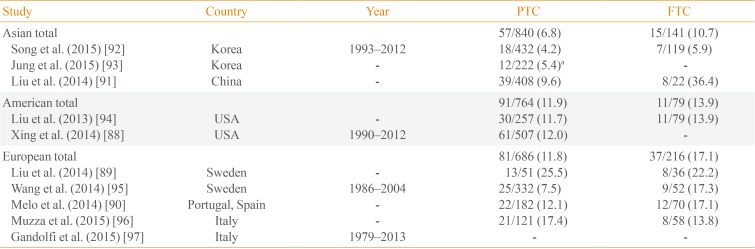
| Study | Country | Year | PTC | FTC |
|---|---|---|---|---|
| Asian total | 57/840 (6.8) | 15/141 (10.7) | ||
| Song et al. (2015) [92] | Korea | 1993-2012 | 18/432 (4.2) | 7/119 (5.9) |
| Jung et al. (2015) [93] | Korea | - | 12/222 (5.4)a | - |
| Liu et al. (2014) [91] | China | - | 39/408 (9.6) | 8/22 (36.4) |
| American total | 91/764 (11.9) | 11/79 (13.9) | ||
| Liu et al. (2013) [94] | USA | - | 30/257 (11.7) | 11/79 (13.9) |
| Xing et al. (2014) [88] | USA | 1990-2012 | 61/507 (12.0) | - |
| European total | 81/686 (11.8) | 37/216 (17.1) | ||
| Liu et al. (2014) [89] | Sweden | - | 13/51 (25.5) | 8/36 (22.2) |
| Wang et al. (2014) [95] | Sweden | 1986-2004 | 25/332 (7.5) | 9/52 (17.3) |
| Melo et al. (2014) [90] | Portugal, Spain | - | 22/182 (12.1) | 12/70 (17.1) |
| Muzza et al. (2015) [96] | Italy | - | 21/121 (17.4) | 8/58 (13.8) |
| Gandolfi et al. (2015) [97] | Italy | 1979-2013 | - | - |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download