Abstract
Neuroendocrine lesions of the thyroid are rare. The most common types are medullary thyroid carcinomas (MTCs) and C-cell hyperplasia. MTCs originate from thyroid parafollicular cells that secrete calcitonin which serves as a serum marker of MTCs. Here, the rare case of a calcitonin-negative neuroendocrine tumor (NET) derived from follicular lesions of the thyroid is described. A 34-year-old man presented at our hospital for the surgical management of an incidental thyroid nodule that was observed on an ultrasound sonography (USG) of the neck. Initially, USG-guided aspiration cytology was performed, and a MTC was suspected. The expressions of thyroglobulin and thyroid transcription factor-1, which are thyroid follicular cell markers, and synaptophysin and chromogranin A, which are neuroendocrine markers, was confirmed following surgical pathology. However, the staining of calcitonin, a marker of MTCs, was not observed. A nonmedullary NET of the thyroid is uncommon, and the distinction between calcitonin-negative NETs and MTCs of the thyroid may be important due to differences in their clinical courses and management.
Thyroid neoplasms are classified into three major categories: epithelial, nonepithelial, and secondary [1234]. Most primary epithelial tumors of the thyroid are derived from follicular cells and include follicular, papillary, medullary, poorly differentiated, and anaplastic carcinomas [1234]. However, neuroendocrine tumors (NETs) of the thyroid, including C-cell lesions, paragangliomas, secondary NETs of the thyroid, and neuroendocrine carcinomas, are extremely rare [1].
A medullary thyroid carcinoma (MTC) is a NET that originates from parafollicular C-cells and can occur in both sporadic and familial forms [5]. MTCs account for approximately 4% to 10% of all thyroid cancers [6,7,8,9] and are unique because of their expression of calcitonin. Elevated calcitonin levels are a highly sensitive and specific indicator of the prognosis and recurrence of a MTC [9,10]. Therefore, calcitonin is considered to be the most useful immunohistochemical marker for the diagnosis of MTCs.
The first case of a calcitonin-negative NET of the thyroid was reported in 2011 [11]. In such cases, a careful interpretation of the immunohistochemical analyses of the tumor is needed, because calcitonin-negative NETs and MTCs may differ in their clinical course and management. Here, the rare case of a calcitonin-negative NET of the thyroid is described.
A 34-year-old man presented at our hospital for the surgical management of an incidental thyroid nodule that was observed on an ultrasound sonography (USG) of the neck. At the clinic, USG-guided fine needle aspiration (FNA) cytology was performed, and a MTC was suspected. The patient was referred to a tertiary hospital for further evaluation where a core needle biopsy was performed and revealed a nonepithelial neural or neuroendocrine malignancy.
The patient did not exhibit any symptoms and did not have a personal or family history of endocrine disorders or a previous history of exposure to radiation. An examination of the neck did not reveal a palpable nodule or lymphadenopathy, and his physical examination was unremarkable. Prior to the operation, the patient's serum levels of calcium, thyroid stimulating hormone, and free thyroxine were within normal ranges. His serum levels of thyroglobulin antigen were 11.64 ng/mL (normal range, 1.4 to 78.0), his thyroid microsomal antibody was negative, and his serum calcitonin level was 3.70 pg/mL (normal range, <10). The patient's carcinoembryonic antigen (CEA) levels were not measured preoperatively, but a solitary hypoechoic nodule of approximately 0.88×0.57×0.88 cm with increased blood flow was observed in the right thyroid following a USG (Fig. 1). A cervical contrast-enhanced computed tomography (CT) scan revealed a low density nodule in the same area. A chest roentgenogram, an electrocardiogram, and positron emission tomography/CT scans did not reveal any abnormalities, and there was no evidence of metastasis to the lymph nodes or other organs. The tentative diagnosis was a NET of the thyroid.
The patient underwent a right lobectomy in March 2012. The specimen measured approximately 0.6×0.5 cm, and the histological findings revealed a poorly differentiated carcinoma with a solid and trabecular pattern; these features are consistent with those of a NET (Fig. 2). There was no vascular invasion or metastasis of the tumor cells into regional lymph nodes. Immunohistochemical analyses were performed on the resected tumor (Fig. 3) and showed that the tumor cells were immunopositive for thyroglobulin and thyroid transcription factor-1 (TTF-1), which are thyroid follicular cell markers, and for synaptophysin and chromogranin A, which are neuroendocrine markers. There were no indications of calcitonin or CEA immunoreactivities in the tumor cells (Table 1). The patient did not exhibit any clinical evidence of tumor recurrence during the 1 year follow-up period.
NETs are neoplasms that originate from cells within the endocrine and nervous systems, and although the majority of NETs are benign, some can be malignant. NETs typically occur in the intestine where they are defined as carcinoid tumors, but they can also occur in the lung and the rest of the body including pancreatic islet cells, the thymus, and parafollicular cells of the thyroid [12]. While NETs have different embryological origins, they have common phenotypical characteristics. For example, NETs are immunopositive for various markers of neuroendocrine differentiation, such as chromogranin A and synaptophysin, and may secrete various peptides and hormones [12]. Neuron-specific enolase is less specific for NETs [12].
Although neuroendocrine lesions of the thyroid are rare, the most common types are MTCs and C-cell hyperplasia [1,13]. Other thyroid nodules and tumors that possess neuroendocrine features include hyalinizing trabecular neoplasms, insular carcinomas, true paragangliomas, parathyroid lesions, and tumors metastatic to the thyroid [13]. MTCs are less common but have a worse prognosis. For example, MTCs tend to spread to lymph nodes very early and therefore require a more aggressive treatment than other types of differentiated thyroid carcinomas, such as papillary and follicular thyroid carcinomas. Although MTC tumor cells can produce CEA, chromogranin A, calcitonin gene-related peptide, adrenocorticotropic hormone, amyloid, somatostatin, serotonin, and vasoactive intestinal peptide, calcitonin is the best indicator for the detection, staging, postoperative management, and prognosis of MTCs [14]. However, the differential diagnosis of a MTC is complicated by the wide spectrum of morphological variants [4].
There are few cases of calcitonin-negative NETs. The first case of a calcitonin-negative nonmedullary NET of the thyroid was reported by Chernyavsky et al. [11]. The tumor in this case expressed follicular cell markers, including thyroglobulin and synaptophysin, and the neuroendocrine marker chromogranin A, but immunohistochemical staining for calcitonin was negative, which is the defining marker of a MTC. In a more recent study, Mussazhanova et al. [15] described the case of a calcitonin-negative small cell neuroendocrine carcinoma which occurred in the thyroid of a patient who had previously been irradiated at a high dose (60 Gy) for pharyngeal cancer; molecular analyses indicated a follicular cell origin. In the present case, the patient had not been exposed previously to radiation; nevertheless, the expression of thyroglobulin, TTF-1, synaptophysin, and chromogranin A were confirmed following surgical pathology. Additionally, although the tumor originated from thyroid follicular cells, it was negative for calcitonin.
The growth patterns of primary thyroid tumors that are not of neuroendocrine origin can sometimes mimic NETs, particularly MTCs. As mentioned by Chernyavsky et al. [11], the differentiation between a MTC and a NET is important, because the prognosis and treatment may be very different. FNA of thyroid nodules is one of the safest and most useful tools available for the diagnosis of thyroid tumors. However, the diagnosis of a MTC using FNA has several drawbacks, and the use of serum calcitonin levels for the diagnosis of MTCs remains controversial [14,16]. Thus, it is important for the physician to be aware and suspicious of nonmedullary NETs when the cells are negative for calcitonin but positive for thyroglobulin. Furthermore, immunohistochemical staining plays a key role in the differentiation of the origin of cells due to pathological limits.
In conclusion, careful interpretation and caution are necessary when tumor cells are negative for calcitonin but positive for thyroglobulin immunohistochemically, because calcitonin-negative NETs and MTCs may differ in their clinical course and management.
Figures and Tables
Fig. 1
Thyroid ultrasound sonography showing an approximately 0.88×0.57×0.88 cm-sized hypoechoic nodule (A, yellow arrow) with increased vascularity (B) of the right lobe.
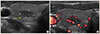
Fig. 2
Histologic findings showing a well-differentiated neuroendocrine tumor (A, H&E stain, ×100) with calcitonin-negative immunohistochemical staining (B, ×200) of tumor cells.
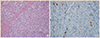
Fig. 3
Immunohistochemistry findings showing strong positive chromogranin A (A, ×100) and synaptophysin (B, ×100) staining of the tumor cells.

References
1. Baloch ZW, LiVolsi VA. Unusual tumors of the thyroid gland. Endocrinol Metab Clin North Am. 2008; 37:297–310.
2. Kovaacs K, Asa SL. Chapter 13, The thyroid gland. Functional endocrine pathology. 2nd ed. Malden: Blackwell Science;1998. p. 295–380.
3. Randolph GW. Surgery of the thyroid and parathyroid glands. 2nd ed. London: Elsevier Health Sciences;2012.
4. Rosai J, Carcangiu ML, DeLellis RA. Armed Forces Institute of Pathology (US). Universities Associated for Research and Education in Pathology. Tumors of the thyroid gland. Washington DC: Armed Forces Institute of Pathology;1992.
5. Griebeler ML, Gharib H, Thompson GB. Medullary thyroid carcinoma. Endocr Pract. 2013; 19:703–711.
6. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Thyroid. 1995; 5:407–424.
7. Marsh DJ, Learoyd DL, Robinson BG. Medullary thyroid carcinoma: recent advances and management update. Thyroid. 1995; 5:407–424.
8. Almeida MQ, Stratakis CA. Solid tumors associated with multiple endocrine neoplasias. Cancer Genet Cytogenet. 2010; 203:30–36.
9. Wells SA Jr, Dilley WG, Farndon JA, Leight GS, Baylin SB. Early diagnosis and treatment of medullary thyroid carcinoma. Arch Intern Med. 1985; 145:1248–1252.
10. Bockhorn M, Frilling A, Rewerk S, Liedke M, Dirsch O, Schmid KW, Broelsch CE. Lack of elevated serum carcinoembryonic antigen and calcitonin in medullary thyroid carcinoma. Thyroid. 2004; 14:468–470.
11. Chernyavsky VS, Farghani S, Davidov T, Ma L, Barnard N, Amorosa LF, Trooskin SZ. Calcitonin-negative neuroendocrine tumor of the thyroid: a distinct clinical entity. Thyroid. 2011; 21:193–196.
12. Langley K. The neuroendocrine concept today. Ann N Y Acad Sci. 1994; 733:1–17.
13. Baloch ZW, LiVolsi VA. Neuroendocrine tumors of the thyroid gland. Am J Clin Pathol. 2001; 115:S56–S67.
14. American Thyroid, Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA Jr. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009; 19:565–612.
15. Mussazhanova Z, Miura S, Stanojevic B, Rougounovitch T, Saenko V, Shiraishi T, Kurashige T, Shichijo K, Kaneko K, Takahashi H, Ito M, Nakashima M. Radiation-associated small cell neuroendocrine carcinoma of the thyroid: a case report with molecular analyses. Thyroid. 2014; 24:593–598.
16. Elisei R, Bottici V, Luchetti F, Di Coscio G, Romei C, Grasso L, Miccoli P, Iacconi P, Basolo F, Pinchera A, Pacini F. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004; 89:163–168.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download