Abstract
Background
Anaplastic thyroid cancer (ATC) is a rare type of thyroid malignancy and one of the most aggressive solid tumors, responsible for between 14% and 50% of the total annual mortality associated with thyroid cancer.
Methods
A retrospective study was made of all ATC cases diagnosed by biopsy in the Philippine General Hospital between 2008 and 2013.
Results
A total of 15 patients were identified, with a median age at diagnosis of 63 years. All tumors were at least 6 cm in size upon diagnosis. All patients had a previous history of thyroid pathology, presenting with an average duration of 11 years. Eleven patients presented with cervical lymphadenopathies, whereas seven exhibited signs of distant metastases, for which the lungs appeared to be the most common site. More than 70% of the patients presented with a rapidly growing neck mass, leading to airway obstruction. Only three patients were treated using curative surgery; the majority received palliative and supportive forms of treatment. In addition, only three patients were offered radiotherapy. Chemotherapy was not offered to any patient. Only two patients were confirmed to still be alive during the study period. The median survival time for the other patients was 3 months; in the majority of cases the patient died within the first year following diagnosis.
Conclusion
Our experience with ATC demonstrated concordance with other institutions with respect to current clinical profile, presentation, and prognosis. An absence of distant metastases and lymph node involvement was associated with improved survival outcomes, whereas age at diagnosis and tumor size did not affect survival. Curative surgery offers the most effective means of prolonging survival. Radiotherapy and chemotherapy in combination with surgery represents a promising treatment strategy.
Anaplastic thyroid cancer (ATC) is a rare type of thyroid malignancy accounting for only 1.3% of all thyroid malignancies [1]. ATC has an estimated incidence of between one and two cases per million population per year [2] and is one of the most aggressive solid tumors in humans, with a median survival time of 4 months following diagnosis [3]. One-year and 10-year survival rates are estimated at between 10% and 20% and at less than 5%, respectively [4]. Despite its rarity, ATC is responsible for between 14% and 50% of the annual mortality associated with thyroid cancer [5].
The incidence of ATC is decreasing worldwide because of increased detection of previously subclinical small papillary thyroid cancers, which increases the incidence of differentiated thyroid carcinomas [6]. The reclassification of small cell ATC as lymphoma or undifferentiated medullary thyroid cancer, together with the fact that insular carcinoma is no longer classified as an ATC variant and the decreased incidence of follicular thyroid cancer from dietary iodine supplementation and earlier diagnosis and treatment of differentiated thyroid cancer, might have eliminated the opportunity to undergo dedifferentiation [2].
ATC is commonly observed in the elderly: the majority of ATC patients are at least 65 to 70 years of age [7]. In addition, the majority of patients present with a rapidly growing neck mass, dysphagia, or voice changes [5] and will have cervical lymph nodes and distant metastases upon diagnosis, automatically rendering ATC as stage 4 [8]. Rapid deterioration and the onset of complications provide physicians with only a brief window in which to intervene. Treatment is generally palliative, although in certain cases a combination of surgery, radiotherapy, and chemotherapy has prolonged survival more effectively [9]. Although the diagnosis and treatment of ATC should be considered medically urgent, end-of-life planning to optimize the quality of the patient's remaining life must also be given a high priority. In certain patients with completely resectable disease, long-term survival has been achieved [10].
Several risk factors for ATC have been identified, including a prior history of thyroid pathology, a family history of thyroid cancer, and iodine deficiency, although definitive associations have proven problematic because of the limited number of cases worldwide [11]. Many prognostic factors have also been identified, including a lesion size <6 cm, less extensive disease and the absence of distant metastases at initial presentation, age <60 years, and female sex [6].
Because of its rarity and the high likelihood of fatality, knowledge regarding the optimal management of ATC remains limited. Currently there is no consensus, first-line treatment modality available worldwide. The majority of the published data are from international studies: in the Philippines, no studies have been published concerning ATC. The present paper thus describes the clinical experience of a tertiary care hospital center in the Philippines (Philippine General Hospital [PGH]) in managing ATC patients.
A retrospective study was made of all ATC cases diagnosed by biopsy in the PGH between 2008 and 2013. To ensure standardization of histopathologic results, only cases in which biopsy was performed in the PGH Department of Pathology were included.
Adult patients (>18 years of age) diagnosed with ATC according to a final histopathologic biopsy report conducted in the PGH Department of Pathology were included.
Patients diagnosed with poorly differentiated squamous cell carcinoma, thyroid lymphoma, metastatic cancer, hurtle cell carcinoma, or insular thyroid carcinoma were excluded.
Both inpatient and outpatient charts of all included patients were retrieved using hospital case numbers via the PGH medical records department. Once retrieved, all charts were carefully reviewed and documented.
Clinical presentation, physical findings, diagnostic tests, management status, clinical course, and eventual outcome were all documented and described. Patients with incomplete data and diagnostic tests were still included in the study and are reported upon accordingly. All data were then tabulated, and descriptive data were subjected to statistical analysis (i.e., mean, median, mode, and standard deviation).
A total of 15 ATC cases were identified based on final histopathologic biopsy results. The sample included eight females (53%) and seven males (47%). The median age at diagnosis was 63 years (range, 36 to 73). All tumors were >6 cm in size upon diagnosis, with a median size of 10 cm (range, 6 to 19). All patients had a previous history of thyroid pathology, with an average duration of 11 years. Nine patients (60%) presented with slow-growing nodular nontoxic goiter, whereas four others (27%) presented initially with colloid adenoma; only two (13%) had a history of papillary thyroid cancer. Eleven patients (73%) presented with cervical lymphadenopathies upon diagnosis; seven (47%) exhibited signs of distant metastases, for which the lungs appeared to be the most common site (Table 1).
More than 70% of the patients presented with a rapidly growing (<3 months), fixed, hard neck mass, rendering airway obstruction the most common reason for admission at our institution. The most frequently presenting signs and symptoms included dysphagia (80%), weight loss (60%), hoarseness (60%), dyspnea (60%), neck pain (40%), cough (33%), superior vena cava syndrome (20%), and hemoptysis (7%). Eleven patients (73%) were chronic smokers, and five (33%) were heavy alcohol drinkers. All patients were clinically euthyroid, as confirmed by an average free T4 of 17.95 pmol/L (normal range, 11 to 24) and a thyroid stimulating hormone level of 1.7 mIU/L (normal range, 0.3 to 3.8).
Fine needle aspiration (FNA) biopsy results were compatible with ATC in only five patients (33%). Two results were suspicious for malignancy, whereas two others indicated papillary thyroid cancer. Six FNA biopsy results (40%) initially demonstrated benign features but were subsequently characterized as ATC according to the final surgical pathologic biopsy.
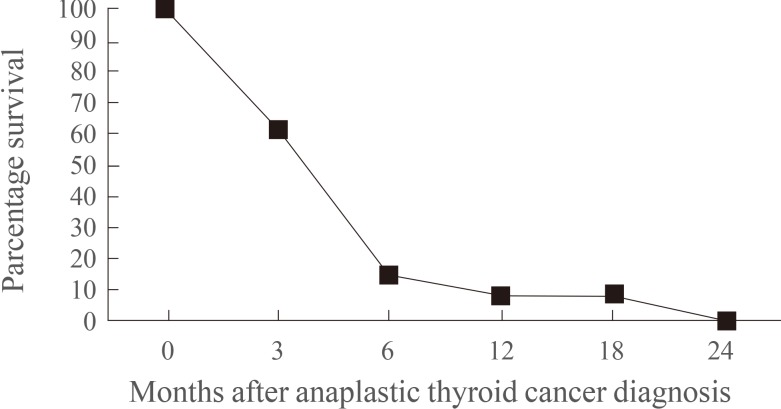
Only three patients (20%) were treated with curative intent via total thyroidectomy with or without radical neck dissection. The majority (80%) of patients received palliative and supportive forms of treatment because of massive metastases upon diagnosis and unresectability of the tumor. Five patients (33%) required an emergency tracheostomy to prevent significant tracheal compression. Only three patients (20%) were offered radiotherapy, but none were able to complete the full regimen. Chemotherapy was not offered to any patient. Only four patients (27%) were referred to hospices, for family meetings and end-of-life care. Only two patients were confirmed as still being alive during the study period (Table 1). The median survival time for the other patients was 3 months from the date of admission and diagnosis. The majority (>90%) of the patients died within the first year following diagnosis (Fig. 1).
Various studies have suggested that ATC originates from a well-differentiated thyroid cancer, subsequently transforming and dedifferentiating into a poorly differentiated tumor, resulting in the loss of cellular features unique to thyroid cells [12]. Papillary thyroid cancer, followed by follicular thyroid cancer, is most frequently predisposed toward anaplastic degeneration [13]. The present study demonstrated that the majority of Filipino patients diagnosed with ATC had a long history of thyroid pathology prior to the onset of rapid tumor growth and metastases, leading to eventual death. Two patients were initially diagnosed with papillary thyroid cancer. The larger tumor sizes (>6 cm) at presentation for the majority of our patients can be attributed to the inaccessibility of medical surveillance and poor follow-up, especially for patients living in rural areas.
Clinically presenting signs and symptoms among our cohort were similar to those in other international studies (Table 2) [7101415]. Although smoking is not reported as a risk factor, the majority (>70%) of the Filipino ATC patients were chronic smokers; it therefore appears that smoking might be associated with the development of ATC, although further studies are required to confirm this. Hormonal overproduction is infrequently observed in papillary thyroid cancer but is seen in follicular thyroid cancer. Hypothyroidism, however, is typically observed in ATC, because the majority of normal thyroid tissue is destroyed by the large compressing tumor.
FNA can diagnose ATC by revealing spindled or giant cells, bizarre neoplastic cells that may be multinucleated, or atypical cells with high mitotic activity. However, histologic confirmation is recommended if the diagnosis is not certain, to exclude tumors with better prognoses or those that require a different therapeutic approach [16]. Several patients referred for ATC based on FNA cytology had reportedly discrepant histologic findings at surgery [17]. In our study, almost 40% of patients had an initially benign FNA biopsy result that was subsequently redefined as ATC following surgery.
Surgical treatment of intrathyroidal tumor offers the best opportunity for prolonged survival. For extrathyroidal tumors, such surgery would be controversial and would confer little benefit in terms of survival. The aggressive behavior of ATC results in high rates of recurrence and potential metastases to distant sites [4]. Resections of neck structures are generally discouraged and would not provide added benefit in terms of mortality [18]. Tracheostomy should be performed in patients with impending airway obstruction in whom death is not imminent from other sites of disease and who are not candidates for local resection or chemoradiation [19]. In the absence of impending risk to the airway, prophylactic tracheostomy is not recommended [16].
Radiation does not alter the course of ATC in the majority of patients. However, when combined with surgery and chemotherapy, it can prolong short-term survival in certain cases. Toxicity can be a limiting factor with radiation [7]. Chemotherapy is another treatment option that can be offered in combination with other modalities, although the majority of cases will still have poor outcomes. Doxorubicin is the most commonly used agent [7]. Bleomycin, etoposide, cisplatin, and methotrexate have poor response rates and no long-term survival benefit for ATC [20]. Although chemotherapy appears unpromising, based on international studies, it might still confer some therapeutic benefit if used in combination with other modalities and should therefore be considered for ATC patients.
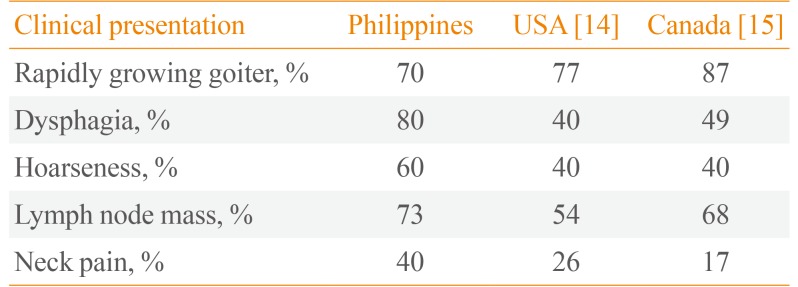
According to the results of several studies, ATC is a disease of the elderly, occurring in individuals with a mean age >60 years. A predominance of female patients has been observed in the majority of the international data but not in our local data. Surprisingly, distant metastases appear to represent the slight minority (i.e., <50%) of cases upon diagnosis in the majority of studies. Surgery remains the most common treatment modality among different institutions; chemotherapy is the least common option (Table 3) [72122232425].
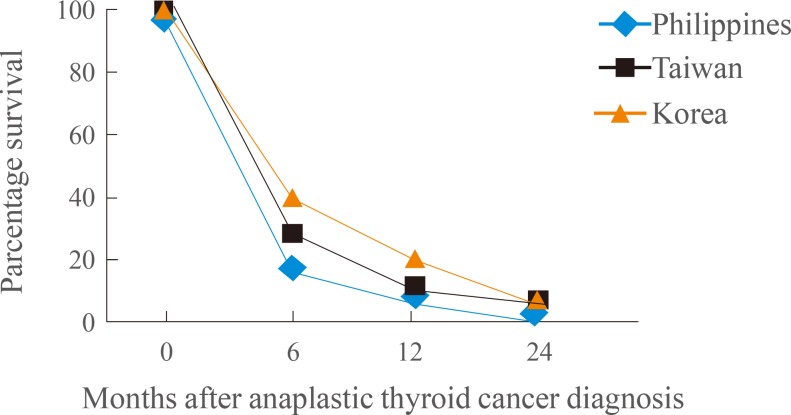
The median survival time among our Filipino cohort (3 months) was shorter than in other studies, which in the majority of cases stated a survival time for ATC of between 5 and 6 months (Fig. 2) [2627]. Younger age (<45 years), a smaller tumor (<6 to 7 cm), the absence of distant metastasis, and extrathyroidal involvement upon diagnosis were good independent prognostic factors in several international studies [28]. However, Haigh and colleagues [9] reported that neither age nor tumor size was associated with survival. Potentially curative surgery was the only variable associated with prolonged survival in their study following multivariate analysis [9]. In the present study, the absence of distant metastases and lymph node involvement during diagnosis, and immediate curative surgical management via total thyroidectomy, were associated with longer survival times (Table 1).
At present, ATC remains invariably fatal worldwide, despite advances in medical care. There is no definitive curative measure and no recommended, optimal treatment regimen; treatment is generally palliative. Combination therapy with surgery, radiotherapy, and chemotherapy appears to prolong survival times in certain cases but is accompanied by side effects. Physicians frequently come into contact with patients too late to effectively manage this type of malignancy, and prognosis is usually very poor upon diagnosis [10].
In conclusion, our experience with ATC demonstrated concordance with other institutions with respect to the current clinical profile, presentation, and prognosis. A minority of female patients and significant associations with smoking history and prior thyroid pathology are unique to our results. An absence of distant metastases and lymph node involvement translated to better survival outcomes, whereas age at diagnosis and tumor size did not affect survival. Curative surgery through total thyroidectomy and aggressive tumor debulking offers the most effective means of prolonging survival. Radiotherapy and chemotherapy in combination with surgery might offer promise for the future.
References
1. Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992-2006. Thyroid. 2011; 21:125–134. PMID: 21186939.

2. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006; 295:2164–2167. PMID: 16684987.

3. Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005; 103:1330–1335. PMID: 15739211.
4. McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001; 130:1028–1034. PMID: 11742333.

5. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer. 1998; 83:2638–2648. PMID: 9874472.
6. Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006; 13:453–464. PMID: 16474910.

7. Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol. 2011; 2011:542358. PMID: 21772843.

8. Agrawal S, Rao RS, Parikh DM, Parikh HK, Borges AM, Sampat MB. Histologic trends in thyroid cancer 1969-1993: a clinico-pathologic analysis of the relative proportion of anaplastic carcinoma of the thyroid. J Surg Oncol. 1996; 63:251–255. PMID: 8982370.

9. Haigh PI, Ituarte PH, Wu HS, Treseler PA, Posner MD, Quivey JM, Duh QY, Clark OH. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001; 91:2335–2342. PMID: 11413523.

10. Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am. 2008; 37:525–538. PMID: 18502341.

11. Bakiri F, Djemli FK, Mokrane LA, Djidel FK. The relative roles of endemic goiter and socioeconomic development status in the prognosis of thyroid carcinoma. Cancer. 1998; 82:1146–1153. PMID: 9506362.

12. Hunt JL, Tometsko M, LiVolsi VA, Swalsky P, Finkelstein SD, Barnes EL. Molecular evidence of anaplastic transformation in coexisting well-differentiated and anaplastic carcinomas of the thyroid. Am J Surg Pathol. 2003; 27:1559–1564. PMID: 14657716.

13. Wallin G, Backdahl M, Tallroth-Ekman E, Lundell G, Auer G, Lowhagen T. Co-existent anaplastic and well differentiated thyroid carcinomas: a nuclear DNA study. Eur J Surg Oncol. 1989; 15:43–48. PMID: 2465188.
14. Giuffrida D, Gharib H. Anaplastic thyroid carcinoma: current diagnosis and treatment. Ann Oncol. 2000; 11:1083–1089. PMID: 11061600.

15. Wang Y, Tsang R, Asa S, Dickson B, Arenovich T, Brierley J. Clinical outcome of anaplastic thyroid carcinoma treated with radiotherapy of once- and twice-daily fractionation regimens. Cancer. 2006; 107:1786–1792. PMID: 16967442.

16. Ain KB. Anaplastic thyroid carcinoma: a therapeutic challenge. Semin Surg Oncol. 1999; 16:64–69. PMID: 9890741.

17. Tennvall J, Lundell G, Wahlberg P, Bergenfelz A, Grimelius L, Akerman M, Hjelm Skog AL, Wallin G. Anaplastic thyroid carcinoma: three protocols combining doxorubicin, hyperfractionated radiotherapy and surgery. Br J Cancer. 2002; 86:1848–1853. PMID: 12085174.

18. Haigh PI. Anaplastic thyroid carcinoma. Curr Treat Options Oncol. 2000; 1:353–357. PMID: 12057160.

19. Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T, Ito K, Ito K, Tanaka S. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. 1999; 84:4043–4049. PMID: 10566647.

20. Koussis H, Scola A, Tonello S. Multimodality therapeutic approach in anaplastic thyroid cancer (ATC): study of 56 patients. J Clin Oncol. 2006; 24(18 Suppl):15533.

21. Sequeiros Santiago G, Llorente Pendas JL, Rodrigo Tapia JP, Puente Verez M, Suarez Nieto C. Anaplastic thyroid carcinoma. Our experience. Acta Otorrinolaringol Esp. 2004; 55:424–429. PMID: 15605808.
22. Lim SM, Shin SJ, Chung WY, Park CS, Nam KH, Kang SW, Keum KC, Kim JH, Cho JY, Hong YK, Cho BC. Treatment outcome of patients with anaplastic thyroid cancer: a single center experience. Yonsei Med J. 2012; 53:352–357. PMID: 22318823.

23. Junor EJ, Paul J, Reed NS. Anaplastic thyroid carcinoma: 91 patients treated by surgery and radiotherapy. Eur J Surg Oncol. 1992; 18:83–88. PMID: 1582515.
24. Veness MJ, Porter GS, Morgan GJ. Anaplastic thyroid carcinoma: dismal outcome despite current treatment approach. ANZ J Surg. 2004; 74:559–562. PMID: 15230790.

25. Besic N, Auersperg M, Us-Krasovec M, Golouh R, Frkovic-Grazio S, Vodnik A. Effect of primary treatment on survival in anaplastic thyroid carcinoma. Eur J Surg Oncol. 2001; 27:260–264. PMID: 11373102.

26. Kim TY, Kim KW, Jung TS, Kim JM, Kim SW, Chung KW, Kim EY, Gong G, Oh YL, Cho SY, Yi KH, Kim WB, Park do J, Chung JH, Cho BY, Shong YK. Prognostic factors for Korean patients with anaplastic thyroid carcinoma. Head Neck. 2007; 29:765–772. PMID: 17274052.

27. Jiang JY, Tseng FY. Prognostic factors of anaplastic thyroid carcinoma. J Endocrinol Invest. 2006; 29:11–17. PMID: 16553028.

28. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. Prognostic factors and therapeutic strategy for anaplastic carcinoma of the thyroid. World J Surg. 2001; 25:617–622. PMID: 11369989.

Table 1
Clinical Characteristics of Patients Diagnosed with Anaplastic Thyroid Cancer
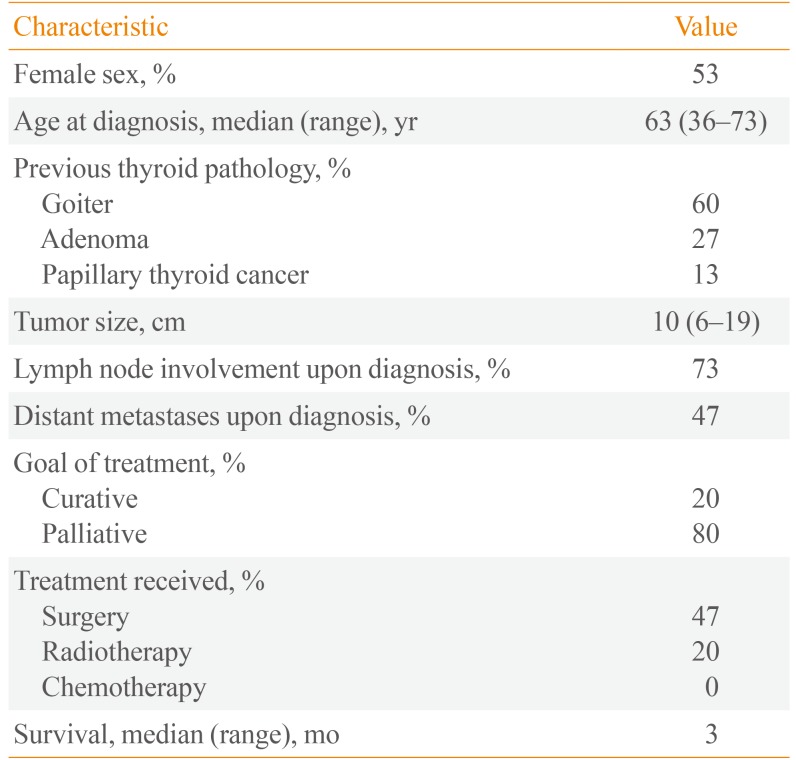
Table 2
Comparison of Clinical Presentation of Anaplastic Thyroid Cancer among International Studies
| Clinical presentation | Philippines | USA [14] | Canada [15] |
|---|---|---|---|
| Rapidly growing goiter, % | 70 | 77 | 87 |
| Dysphagia, % | 80 | 40 | 49 |
| Hoarseness, % | 60 | 40 | 40 |
| Lymph node mass, % | 73 | 54 | 68 |
| Neck pain, % | 40 | 26 | 17 |
Table 3
Comparison of the Clinical Profiles and Treatment Modalities of Patients with Anaplastic Thyroid Cancer among Different International Studies
| Variable |
Philippines (n=15) |
USA [7] (n=11) |
Spain [21] (n=15) |
Korea [22] (n=13) |
UK [23] (n=91) |
Australia [24] (n=18) |
Slovenia [25] (n=162) |
|---|---|---|---|---|---|---|---|
| Age at diagnosis, yr | 60 | 69 | 74 | 69 | 70 | 72 | 65 |
| Female sex, % | 53 | 64 | 73 | 69 | 71 | 67 | 67 |
| Metastases upon diagnosis, % | 47 | 73 | 27 | 46 | 34 | 28 | 51 |
| Treatment received, % | |||||||
| Surgery | 47 | 73 | 40 | 62 | 98 | 78 | 42 |
| Radiotherapy | 20 | 73 | 33 | 62 | 95 | 56 | 31 |
| Chemotherapy | 0 | 45 | 0 | 38 | 0 | 28 | 27 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download