Abstract
Ectopic adrenocorticotropic hormone (ACTH) syndrome is caused most frequently by a bronchial carcinoid tumor or by small cell lung cancer. Medullary thyroid carcinoma (MTC) is a rare etiology of ectopic ACTH syndrome. We describe a case of Cushing syndrome due to ectopic ACTH production from MTC in a 48-year-old male. He was diagnosed with MTC 14 years ago and underwent total thyroidectomy, cervical lymph node dissection and a series of metastasectomies. MTC was confirmed by the pathological examination of the thyroid and metastatic mediastinal lymph node tissues. Two years after his last surgery, he developed Cushingoid features, such as moon face and central obesity, accompanied by uncontrolled hypertension and new-onset diabetes. The laboratory results were compatible with ectopic ACTH syndrome. A bilateral adrenalectomy improved the clinical and laboratory findings that were associated with Cushing syndrome. This is the first confirmed case of ectopic ACTH syndrome caused by MTC in Korea.
Medullary thyroid carcinoma (MTC) is a rare disease that accounts for 3% of thyroid cancers [1]. Most MTCs are nonfunctioning, but in rare cases they secrete adrenocorticotropic hormone (ACTH) or corticotropin-releasing hormone (CRH), causing ectopic ACTH syndrome, one of the causes of Cushing syndrome. Approximately 0.6% of patients with MTC have ectopic ACTH syndrome [2]; and MTC accounts for 2% to 7.5% of ectopic ACTH syndrome [2,3,4].
Globally, approximately 50 cases have been reported about ectopic ACTH syndrome induced by MTC [5], but there have been none from Korea. The authors report the first case in Korea of the patient with MTC that induces ectopic ACTH syndrome in disease progression.
A 48-year-old male was referred to the endocrinology clinic complaining of generalized edema, increased blood pressure and blood glucose levels. The patient was on medication due to hypertension that was diagnosed 1 year ago. Recently, the blood pressure was not well-controlled, the blood glucose level was high and generalized edema developed.
The patient was diagnosed with MTC 14 years ago; total thyroidectomy with right cervical lymph node dissection was performed and I-131 metaiodobenzylguanidine (MIBG) 200 mCi was administered. He was treated with tumor resection for recurred MTC on a mediastinal lymph node 13 years ago. Nine years ago, MTC recurred on the left cervical and left parahilar lymph nodes and was treated with I-131 MIBG 200 mCi after surgery. Two years ago, MTC recurred thirdly on the cervical and mediastinal lymph nodes and lung and was treated with cervical and mediastinal lymph node dissection and the left upper lobe wedge resection of the lung. Since then, the patient has not shown any signs or symptoms of recurrence.
The patient's height was 172 cm and weight was 84 kg, 2 kg over his usual weight. His blood pressure was 185/110 mm Hg with a pulse rate of 90 beats per minute and a breathing rate of 18 breaths per minute. The patient's body temperature was 37℃. Moon face and facial flushing were observed. Buffalo hump and purple striae were absent. Mild pitting edema was observed on the anterior tibia and slight skin pigmentation was present.
Peripheral blood examination showed a white blood cell count of 14,450 cells/µL, hemoglobin of 15.3 g/dL, and platelet count of 358,000 cells/µL. On serum biochemical examination, total cholesterol was 252 mg/dL with total protein of 6.5 g/dL, albumin of 4.1 g/dL, total bilirubin of 1.1 mg/dL, alkaline phosphatase of 101 U/L, aspartate aminotransferase of 17 U/L, alanine aminotransferase of 62 U/L, blood urea nitrogen of 10.3 mg/dL, and creatinine of 0.6 mg/dL. On serum electrolyte examination, sodium was 145 mmol/L with potassium of 2.8 mmol/L, chloride of 94 mmol/L, and bicarbonate of 40.4 mmol/L. Fasting plasma glucose level was 168 mg/dL with glycated hemoglobin of 8.6%. Hormone examination showed total T4 of 12.75 µg/dL (range, 5 to 13) and thyroid stimulating hormone of 0.07 µIU/mL (range, 0.15 to 5.0). Serum calcitonin was 127.86 pg/mL (<10.0) and carcinoembryonic antigen was 29.46 ng/mL (<5). Plasma ACTH concentration was measured with immunoradiometric assay (ELSA-ACTH kit, CisBio, Belford, MA, USA), and was 203.9 pg/mL (range, 6.0 to 60.0). Serum cortisol concentration was 36.0 µg/dL (range, 5 to 25.0) with a 24-hour urine cortisol of 2,237.1 µg/day (range, 20 to 90). On the low dose dexamethasone suppression test, inhibited serum cortisol was 32.50 µg/dL. The 24-hour urine examination results were as follows: metanephrine 14.46 µg/day (range, 52 to 341), normetanephrine 28.21 µg/day (range, 88 to 444), epinephrine 6.57 µg/day (<40), norepinephrine 26.43 µg/day (<80), and vanillylmandelic acid 3.51 mg/day (<8). To validate the RET gene mutation, peripheral blood was collected from the patient and white blood cells were separated by centrifugation to extract genomic DNA. For polymerase chain reaction, BIGDYE version 3.1 cycles sequencing kit (Applied Biosystems, Foster, CA, USA) reagent was utilized and 3,730 automated sequencer (Applied Biosystems) was used to analyze DNA sequence. The DNA sequencing showed no RET gene mutation.
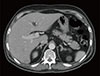
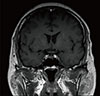
Chest and abdomen computed tomography revealed recurred lesions on the mediastinal lymph nodes, lungs, and liver (Fig. 1), but no abnormal findings in adrenal glands (Fig. 2). Sellar magnetic resonance imaging showed no abnormality in the pituitary gland (Fig. 3). Gastroscopy, colonoscopy, and positron emission tomography did not identify suspicious primary lesions in other regions.
Calcitonin and chromogranin A staining was positive on resected tissues on the third recurrence 2 years ago, which is indicative of MTC. ACTH immunohistochemistry staining was negative (Fig. 4).
Because curative resection of the MTC lesions was difficult on the fourth recurrence, close follow-up with further chemotherapy was considered. To control the ectopic ACTH syndrome, the patient was administered ketoconazole. The dosage was gradually increased over 3 months up to the maximum dosage (1,200 mg/day), but the serum cortisol concentration level did not decrease. Therefore, bilateral adrenalectomy was performed. After surgery, ACTH remained above 200 pg/mL, but plasma glucose and blood pressure levels were improved sufficiently and the oral antidiabetic agent and antihypertensive medication were discontinued. The patient has been living in good condition until 6 months after the surgery without any medication other than glucocorticoid and mineralocorticoid agents.
Ectopic ACTH syndrome is diagnosed when Cushing syndrome occurs with ACTH or CRH secretion from another origin than the pituitary gland or hypothalamus and accounts for 9% to 18% of Cushing syndrome cases [6]. The most common diseases that cause ectopic ACTH syndrome are endobronchial carcinoid tumor and small cell lung cancer and, rarely, MTC can be another source, as reported in this case.
The onset of ectopic ACTH syndrome varies in patients with MTC [2]. Ectopic ACTH syndrome can be detected before or up to 20 years after MTC is diagnosed [7]. The present case describes the patient diagnosed with ectopic ACTH syndrome on the fourth recurrence, 14 years later from the first diagnosis of MTC. In most cases, ectopic ACTH syndrome can occur when MTC has progressed substantially [8], as shown in this case where MTC spread with multiple distant metastases on the mediastinal lymph nodes, lungs, and liver.
It has been reported that different origins of ectopic ACTH syndrome can cause different symptoms [3]. Especially, neuroendocrine carcinoma more commonly accompanies hypertension, weight increase, acne, weakness, and neuropsychiatric symptoms than hypokalemia, skin pigmentation, and edema.
In the present case, we could not obtain a tissue from the first resected thyroid cancer. Therefore, ACTH staining was performed on mediastinal lymph node tissue obtained from the third recurrence, with negative results. Negative ACTH immunoreactivity despite evidence for tumors ACTH production has previously been reported and it has been suggested to reflect reduced ACTH storage concentrations due to a high secretion rate [9]. An alternative explanation could be inefficient translation and processing of pro-opiomelanocortin (POMC) mRNA [9]. Recently, a method was devised to confirm POMC mRNA using in situ hybridization [10]. Some researchers suggested that POMC mRNA in situ hybridization on MTC tissue before clinical signs and symptoms appear could predict the expression of ectopic Cushing syndrome [10]. Additionally, a method confirming ACTH correction after tumor removal or measuring ACTH level around the tumor through catheterization can help diagnose ectopic ACTH syndrome. However, in our case, as the tumor tissue could not be obtained after ectopic ACTH syndrome was diagnosed, POMC mRNA in situ hybridization and ACTH measurement before and after surgery could not be performed. Also, ACTH level around metastatic lesion could not be measured because catheter insertion was technically difficult.
In the present case, the patient suffered from secondary hypertension and diabetes due to ectopic ACTH syndrome and complained of fatigue and weakness. Hypertension is prevalent in approximately 80% of endogenous Cushing syndrome patients and is more common in ectopic ACTH syndrome patients, about 95% [11]. Hypertension in hypercortisolism can be explained by mechanisms such as the glucocorticoid effect of cortisol, activation of renin-angiotensin system, and the cortisol effect in blood vessels [12].
Since a large number of patients with Cushing syndrome die from cardiovascular disorders, controlling hypertension is important. However, controlling blood pressure without treating Cushing syndrome is difficult. Diabetes mellitus is also common complication for Cushing syndrome patients who are chronically exposed to glucocorticoids and diabetes is another factor related to patient mortality. Uncontrolled Cushing syndrome can induce infections and thrombosis, which increase mortality [13]. In this case, the patient was diagnosed with not only hypertension but also diabetes, and therefore, had a higher risk of cardiovascular disorders. In order to control Cushing syndrome, surgical removal of the MTC secreting ACTH ectopically is recommended, but in many cases that is not possible. The alternative is to treat with medication or perform bilateral adrenalectomy [8]. Agents controlling hypercortisolism in Cushing syndrome include ketoconazole, metyrapone, somatostatin analog, and etomidate. Ketoconazole is readily available and has high efficacy [14]; in our case, ketoconazole was administered for 3 months. However, because no significant effect was achieved, bilateral adrenalectomy was performed.
Currently, no effective treatments for MTC with distant metastasis are available. External beam radiotherapy has limited effect and cytotoxic chemotherapy doesn't show any significant survival gain. Tyrosine kinase inhibitors directed against RET such as motesanib, sorafenib, and sunitinib were introduced and had an effect on some patients, but the results were below expectation. Recently, vandetanib (brand name, Zactima) was approved by the U.S. Food and Drug Administration and will soon be introduced in Korea. Vandetanib is known to control epidermal growth factor receptor and vascular endothelial growth factor, and is also effective in patients with and without RET mutation [15]. In the present case, the patient was treated conservatively with close follow-up. This patient may require further treatment for MTC later, and vandetanib may be an option.
MTC rarely secretes ACTH or CRH causing ectopic ACTH syndrome; only 0.6% of patients with MTC reportedly have ectopic ACTH syndrome, but no cases have been reported in Korea. The authors experienced a patient who was diagnosed 14 years previously with MTC, treated with surgery and radioactiveiodine with repeating recurrence and then ectopic ACTH syndrome occurred as the disease progressed substantially. Depending on clinical progress, MTC can induce ectopic ACTH syndrome. The present case demonstrates that proper hormonal evaluation and treatment are necessary for patients with MTC.
Figures and Tables
References
1. Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000; 88:1139–1148.
2. Barbosa SL, Rodien P, Leboulleux S, Niccoli-Sire P, Kraimps JL, Caron P, Archambeaud-Mouveroux F, Conte-Devolx B, Rohmer V. Groupe d'Etude des Tumeurs Endocrines. Ectopic adrenocorticotropic hormone-syndrome in medullary carcinoma of the thyroid: a retrospective analysis and review of the literature. Thyroid. 2005; 15:618–623.
3. Isidori AM, Kaltsas GA, Pozza C, Frajese V, Newell-Price J, Reznek RH, Jenkins PJ, Monson JP, Grossman AB, Besser GM. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab. 2006; 91:371–377.
4. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005; 90:4955–4962.
5. Sand M, Uecker S, Bechara FG, Gelos M, Sand D, Wiese TH, Mann B. Simultaneous ectopic adrenocorticotropic hormone syndrome and adrenal metastasis of a medullary thyroid carcinoma causing paraneoplastic Cushing's syndrome. Int Semin Surg Oncol. 2007; 4:15.
6. Wajchenberg BL, Mendonca BB, Liberman B, Pereira MA, Carneiro PC, Wakamatsu A, Kirschner MA. Ectopic adrenocorticotropic hormone syndrome. Endocr Rev. 1994; 15:752–787.
7. Chrisoulidou A, Pazaitou-Panayiotou K, Georgiou E, Boudina M, Kontogeorgos G, Iakovou I, Efstratiou I, Patakiouta F, Vainas I. Ectopic Cushing's syndrome due to CRH secreting liver metastasis in a patient with medullary thyroid carcinoma. Hormones (Athens). 2008; 7:259–262.
8. Mure A, Gicquel C, Abdelmoumene N, Tenenbaum F, Francese C, Travagli JP, Gardet P, Schlumberger M. Cushing's syndrome in medullary thyroid carcinoma. J Endocrinol Invest. 1995; 18:180–185.
9. Kristiansen MT, Rasmussen LM, Olsen N, Asa SL, Jorgensen JO. Ectopic ACTH syndrome: discrepancy between somatostatin receptor status in vivo and ex vivo, and between immunostaining and gene transcription for POMC and CRH. Horm Res. 2002; 57:200–204.
10. Sheikh-Ali M, Krishna M, Lloyd R, Smallridge RC. Predicting the development of Cushing's syndrome in medullary thyroid cancer: utility of proopiomelanocortin messenger ribonucleic acid in situ hybridization. Thyroid. 2007; 17:631–634.
11. Stewart PM, Walker BR, Holder G, O'Halloran D, Shackleton CH. 11 beta-Hydroxysteroid dehydrogenase activity in Cushing's syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995; 80:3617–3620.
12. Fraser R, Davies DL, Connell JM. Hormones and hypertension. Clin Endocrinol (Oxf). 1989; 31:701–746.
13. Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J Clin Endocrinol Metab. 2000; 85:42–47.
14. Winquist EW, Laskey J, Crump M, Khamsi F, Shepherd FA. Ketoconazole in the management of paraneoplastic Cushing's syndrome secondary to ectopic adrenocorticotropin production. J Clin Oncol. 1995; 13:157–164.
15. Wells SA Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010; 28:767–772.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download