Abstract
Background
The effect of caloric restriction (CR) in the setting of diabetes on bone metabolism has not yet been fully studied. The aim of this study is to determine if short-term CR alters bone mass and metabolism in Otsuka Long-Evans Tokushima fatty (OLETF) rats, an animal model of type 2 diabetes.
Methods
Four groups (n=5) were created: OLETF rats with food ad libitum (AL), OLETF rats with CR, Long-Evans Tokusima Otsuka (LETO) rats with food AL, and LETO rats with CR. The CR condition was imposed on 24-week-old male rats using a 40% calorie reduction for 4 weeks. The effect of CR on femoral bone mineral density (BMD) was assessed by dual-energy X-ray absorptiometry. Serum markers were measured by immunoassay.
Results
After 4 weeks of CR, body weight decreased in both strains. The BMD decreased in LETO rats and was maintained in OLETF rats. After adjustment for body weight, BMD remained lower in LETO rats (P=0.017) but not OLETF rats (P=0.410). Bone-specific alkaline phosphatase levels decreased in LETO rats (P=0.025) but not in OLEFT rats (P=0.347). Serum leptin levels were reduced after CR in both strains, but hyperleptinemia remained in OLETF rats (P=0.009). CR increased 25-hydroxyvitamin D levels in OLETF rats (P=0.009) but not in LETO rats (P=0.117). Additionally, interleukin-6 and tumor necrosis factor-α levels decreased only in OLETF rats (P=0.009).
Caloric restriction (CR) consistently extends the lifespan of animals, regardless of species or sex, and significantly reduces the incidence of numerous age-related diseases and lesions [1]. Despite the beneficial aspects of CR in terms of mortality and disease, diet affects bone health [2] and CR paradigms may adversely affect bone physiology and mechanics.
Type 2 diabetes mellitus (T2DM) is caused by resistance to insulin and inadequate compensatory insulin secretion in response to glucose [3]. It is commonly associated with obesity, which has been shown to be a good predictor of bone mineral density (BMD). However, there are conflicting reports on the association between BMD and T2DM. Different researchers have observed increased BMD [4,5,6,7,8] and no association or decreased BMD [9,10,11,12,13,14,15].
In patients with early T2DM, lifestyle modifications, including diet-control measures such as short-term CR, are common treatment methods. However, it is not known if short-term CR alters BMD in patients with early T2DM. To study the relationship between BMD and CR we chose the Otsuka Long-Evans Tokushima fatty (OLETF) rat as an animal model of T2DM. The characteristic features of OLETF rats are: 1) late-onset hyperglycemia, 2) a chronic course of diabetes, 3) mild obesity, and 4) early-stage hyperinsulinemia. The OLETF rat develops diabetes spontaneously from 24 weeks of age, which is then followed by the development of several diabetic complications [16]. Therefore, the OLETF rat is suitable for early T2DM animal models at 24-weeks of age. This study was designed to clarify whether bone mass and metabolism are affected by short-term CR in rats with early T2DM.
Animal experiments were approved by the University Ethics Committee and local authorities and were conducted according to official guidelines.
Male OLETF (early T2DM) and Long Evans Tokusima Otsuka (LETO; control) rats aged 5 weeks were obtained from Tokushima Research Institute Otsuka Pharmaceutical Co., Ltd. (Tokushima, Japan). The rats were divided into four groups according to the following conditions: OLEFT rats fed ad libitum (AL; OLETF-AL group, n=5), OLETF rats with CR (OLETF-CR group, n=5), LETO rats fed AL (LETO-AL group, n=5), and LETO rats with CR (LETO-CR group, n=5). CR was started at 24 weeks of age when OLETF rats develop diabetes spontaneously. We weaned OLETF and LETO rats onto a 40% calorie-restricted or normal diet. AL groups were fed a standard laboratory solid diet formulation (AIN-76A Purified Rodent Diet) and CR group were fed the same diet at 60% of the normal AL consumption for 4 weeks. Each animal was housed in a separate cage with free access to water and a 12-hour light/dark cycle (lights on at AM 7:00 and off at PM 7:00). Food intake and body weight were recorded daily.
We measured the BMD of all rats at baseline and after the 4-week dietary intervention (AL or CR). The BMD values for the right femur were measured in vivo by dual-energy X-ray absorptiometry (DXA) using a Lunar Prodigy densitometer (GE Medical, Madison, WI, USA) calibrated daily using a standard phantom provided by the manufacturer. The DXA scanner was set up for the analysis of small animals. All analyses were conducted by high-precision DXA with a 1-mm scan pitch and the region of interest was the whole femur. For each scan, the rat was placed in a supine position with the hind legs maintained in abduction by taping the feet to the pelvis with the tibia placed at a 45° angle to the femur. DXA measurements were repeated three times without repositioning and the values obtained were averaged. The coefficient of variation for the total hip was less than 1%.
All animals were dissected under ether anesthesia at the age of 28 weeks. A fasting blood sample was taken from the abdominal aorta of each rat. Each blood sample was centrifuged at 2,500×g for 15 minutes to extract serum and all serum samples were stored at -70℃. We measured serum leptin (Crystal Chem Inc., Downers Grove, IL, USA), adiponectin (Cusabio Biotech Co., Ltd., Hubei, China), C-telopeptide of type-1 collagen (CTX, Cusabio Biotech Co., Ltd.), insulin (Mercodia AB, Uppsala, Sweden), and 25-hydroxy vitamin D (25(OH)D, ALPCO Diagnostics, Salem, NH, USA) using enzyme-linked immunosorbent assays. Levels of bone-specific alkaline phosphatase (BSAP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were measured by an antibody detection method (Millipore, Billerica, MA, USA).
Data were analyzed and assessed using the SPSS software version 18.0 (IBM Co., Armonk, NY, USA). For all variables, descriptive statistics, including mean and standard deviations of each group, were determined. Differences in all parameters between the four groups were assessed by the Mann-Whitney U test. Differences in all parameters between baseline and postintervention were assessed by the Wilcoxon signed rank test. To compare the BMD values adjusted by weight, we used the analysis of covariance. A P<0.05 was considered to indicate statistical significance.
At 24 weeks of age, we measured the baseline body weight and glucose levels in each group. At the time of diet allocation, we confirmed the diabetic status of OLETF rats by confirming the presence of higher glucose levels compared to LETO rats (P=0.009). For both strains, baseline characteristics did not differ between the AL and CR groups.
In LETO rats, there was no significant difference between baseline and postintervention serum glucose levels in either group (P=0.176 in the AL group and P=0.465 in the CR group). After 4 weeks, there was no difference in serum glucose levels between the AL and CR groups (P=0.170). Body weight increased after 4 weeks of AL feeding (P=0.043) but decreased in the CR group (P=0.043) compared with baseline. After the 4-week dietary intervention, body weight was significantly different between the AL and CR groups (P=0.001). After the 4-week dietary intervention, femur BMD tended to be higher compared to baseline in the AL group (0.264±0.008 vs. 0.275±0.013; P=0.068) and significantly decreased in the CR group (0.259±0.006 vs. 0.216±0.004; P=0.039). After adjustment for body weight, femur BMD in LETO rats was significantly lower in the CR group compared to the AL group (0.276±0.010 vs. 0.215±0.001; P=0.017).
In OLETF rats, serum glucose levels and body weight increased from baseline in the AL group (P=0.043) but decreased in the CR group (P=0.043). The 4-week dietary intervention caused significant differences in serum glucose levels and body weight between the AL and CR groups (P=0.009). After the 4-week dietary intervention, femur BMD increased slightly in the AL group (P=0.068) but not in the CR group (P=0.713). Contrary to findings in the LETO rats, after adjustment for weight, femur BMD tended to be higher in the CR group but this finding was not statistically significant (P=0.401) (Table 1, Fig. 1). After 4 weeks of CR, the change of BMD in LETO of CR group and those in OLETF of CR group was significantly different with repeated measure analysis of variance (P=0.013). At the end of the study time, femur BMD increased under CR conditions only in OLETF rats.
CR lowered leptin levels in both rat strains (P=0.009) and increased adiponectin levels in OLETF rats (P=0.009) but not LETO rats. Levels of the bone formation marker BSAP were decreased after CR in LETO rats (P=0.027) but not in OLETF rats (P=0.347). CR did not affect levels of CTX, a well-known bone resorption marker, in either strain. After CR conditions, 25(OH)D levels were significantly increased in OLETF rats (P=0.009) but not in LETO rats (P=0.117). Levels of the inflammatory markers IL-6 and TNF-α were significantly lowered after CR in OLETF rats (P=0.009) but not in LETO rats (Table 2, Fig. 2).
It is well known that BMD and bone strength can be predicted by body mass. However, our data showed that short-term CR does not lead to bone loss in rats with T2DM, despite weight loss. We thought bone loss that accompanies weight loss is not simply attributable to a decrease in load bearing by the skeleton and is likely affected by many other factors such as endocrine disease, hormonal changes, nutrition, and inflammation. Bone metabolism affected by the adipocyte hormones leptin and adiponectin has been extensively studied. Plasma leptin is 16-kDa protein hormone that plays a key role in regulating energy intake and expenditure, including appetite and hunger, metabolism, and behavior. It increases with weight gain and falls during CR [17]. The relationship between leptin and bone metabolism is complex and previous studies have reported negative [18], positive [19,20,21,22], and no correlations [23,24]. Hamrick et al. [25] suggested that the drop in leptin levels with CR leads to reduced bone mass in mice, which is consistent with other studies showing that leptin treatment prevents reductions in bone formation marker levels during conditions of nutritional stress [26,27]. In our study, leptin levels decreased after CR in both strains but the absolute level of leptin remained higher in OLETF rats. This likely explains why OLETF rats subjected to CR did not lose bone mass or show reductions in the levels of bone formation markers. On the other hand, BMD decreased in LETO rats with reduction in leptin levels owing to their higher sensitivity than in OLETF rats.
Adiponectin is another adipocyte hormone that might have an effect on bone under search. Even though no direct comparison regarding the effect of CR on adiponectin levels between type 2 diabetes and control has been performed, there are some conflicting reports discussing the relationship between adiponectin and BMD. A few studies reported a negative association between adiponectin and bone [28,29] while others reported a positive association. In previous studies, adiponectin was shown to stimulate osteoblast proliferation in vitro [30] and adenoviral overexpression in mice promoted bone formation in vivo [31]. Thus, it is suggested that an increase in adiponectin with weight loss might be expected to have a stimulatory effect on bone. In this study, adiponectin was unchanged in LETO rats and was increased in OLETF rats. This may be an explanation as to why BMD did not decrease in OETF rats. Even though evidence for associations between circulating adipose hormone concentrations and BMD or bone turnover markers has been contradictory, there is evidence that reported signaling by adipokines, such as leptin, may be involved in the suppression of bone formation under conditions of CR [32]. One study showed that leptin prevents the fall in plasma osteocalcin during starvation in male mice [26] and Tatsumi et al. [32] demonstrated that life-long CR suppresses bone formation in C57BL/6 mice, but not in db/db mice. Likewise, in this study, BSAP levels decreased in LETO rats, but not in OLETF rats, after short-term CR. The exact mechanism by which CR suppresses bone formation has not yet been clarified. However, a possible mechanism that has been suggested is peripheral body fat may regulate bone metabolism through systemic circulating factors in addition to its effects on bone mediated by load-bearing [33].
A recent study showed that prolonged CR is associated with lower BMD in lean female rats compared to obese rats [34]. One of the possible explanations was that energy restriction is less detrimental to bone in obese rats compared with lean rats, possibly owing to the fact that conditions of CR increased vitamin D levels in obese rats. This can be explained by obesity, a state of body fat expansion and insulin resistance, and has been found to be associated with low levels of serum 25(OH)D [35,36]. The inverse correlation between obesity and 25(OH)D levels is mainly attributed to the sequestration of this fat-soluble vitamin in adipose tissue. We found the same pattern of vitamin D levels which were decreased in OLETF rats and increased by CR. Increase of vitamin D is not inevitable for increment of BMD and could be one of the reasons BMD was maintained.
There are some studies explaining how systemic inflammation, including T2DM and obesity, affects the bone metabolism. The production of IL-1, IL-6, and/or TNF-α correlated positively with bone loss not only in healthy subjects [37] but also in those with nonimmunological chronic diseases, including diabetes [38]. CR reduces the levels of many inflammatory mediators [39,40], suggesting a link between energy status and inflammation. In our study, after 4 weeks of CR, IL-6, and TNF-α levels were significantly decreased in OLETF rats only. This is a plausible explanation as to why OLETF rats lost weight but maintained bone mass.
This study has several limitations. First, the DXA method used was not specifically designed for animals even though we used software designed specifically for small animals. However, differences in variables are statistically significant because we applied the same DXA method to all experimental groups. Moreover, we measured BMD three times in each animal and used the mean value. Second, we did not evaluate bone strength and bone mass in vitro. Third, we did not determine parathyroid hormone levels and calcium status in the rats.
Nevertheless, to our knowledge, this is the first study to compare the short-term effects of CR on bone in OLETF rats, an animal model of early T2DM. CR decreased the BMD in LETO rats and had no effect in OLETF rats. Based on our results, it is conceivable that short-term CR may not alter bone mass and bone metabolism in patients with T2DM. Clinical studies of the efficacy of CR in patients with early T2DM may thus be warranted in the future.
Figures and Tables
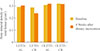 | Fig. 1After 4 weeks of caloric restriction (CR), the bone mineral density of the whole femur decreased in Long-Evans Tokushima Otsuka (LETO) rats and was maintained in Otsuka Long-Evans Tokushima fatty (OLETF) rats. AL, ad libitum. aP<0.05 compared with baseline. |
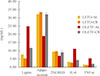 | Fig. 2After 4 weeks of caloric restriction (CR), leptin levels decreased in Long-Evans Tokushima Otsuka (LETO) and Otsuka Long-Evans Tokushima fatty (OLETF) rats. In OLETF rats, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels decreased after CR, while 25-hydroxyvitamin D (25(OH)D) levels increased. AL, ad libitum. aP<0.01 compared with LETO-AL rats; bP<0.01 compared with OLETF-AL rats. |
Table 1
Baseline Characteristics and Bone Mineral Density Changes after 4 Weeks of Caloric Restriction

Table 2
Adipokines, Bone Turnover Markers, Inflammatory Markers, and 25-Hydroxyvitamin D Levels after 4 Weeks of Caloric Restriction

Values are expressed as mean±SD. P<0.05 was considered to indicate statistical significance.
LETO, Long-Evans Tokushima Otsuka; AL, ad libitum; CR, caloric restriction; OLETF, Otsuka Long-Evans Tokushima fatty; BSAP, bone-specific alkaline phosphatase; CTX, C-telopeptide of type 1 collagen; 25(OH)D, 25-hydroxyvitamin D; IL-6; interleukin-6; TNF-α, tumor necrosis factor-α.
References
1. Lipman RD, Dallal GE, Bronson RT. Effects of genotype and diet on age-related lesions in ad libitum fed and calorie-restricted F344, BN, and BNF3F1 rats. J Gerontol A Biol Sci Med Sci. 1999; 54:B478–B491.
2. Anderson JJ, Rondano P, Holmes A. Roles of diet and physical activity in the prevention of osteoporosis. Scand J Rheumatol Suppl. 1996; 103:65–74.
3. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003; 26:Suppl 1. S5–S20.
4. Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR. Study of Osteoporotic Features Research Group. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001; 86:32–38.
5. van Daele PL, Stolk RP, Burger H, Algra D, Grobbee DE, Hofman A, Birkenhager JC, Pols HA. Bone density in non-insulin-dependent diabetes mellitus. The Rotterdam Study. Ann Intern Med. 1995; 122:409–414.
6. Bauer DC, Browner WS, Cauley JA, Orwoll ES, Scott JC, Black DM, Tao JL, Cummings SR. Factors associated with appendicular bone mass in older women. The Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1993; 118:657–665.
7. Christensen JO, Svendsen OL. Bone mineral in pre- and postmenopausal women with insulin-dependent and non-insulin-dependent diabetes mellitus. Osteoporos Int. 1999; 10:307–311.
8. Lunt M, Masaryk P, Scheidt-Nave C, Nijs J, Poor G, Pols H, Falch JA, Hammermeister G, Reid DM, Benevolenskaya L, Weber K, Cannata J, O'Neill TW, Felsenberg D, Silman AJ, Reeve J. The effects of lifestyle, dietary dairy intake and diabetes on bone density and vertebral deformity prevalence: the EVOS study. Osteoporos Int. 2001; 12:688–698.
9. Levin ME, Boisseau VC, Avioli LV. Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med. 1976; 294:241–245.
10. Ishida H, Seino Y, Matsukura S, Ikeda M, Yawata M, Yamashita G, Ishizuka S, Imura H. Diabetic osteopenia and circulating levels of vitamin D metabolites in type 2 (non-insulin-dependent) diabetes. Metabolism. 1985; 34:797–801.
11. Giacca A, Fassina A, Caviezel F, Cattaneo AG, Caldirola G, Pozza G. Bone mineral density in diabetes mellitus. Bone. 1988; 9:29–36.
12. Weinstock RS, Goland RS, Shane E, Clemens TL, Lindsay R, Bilezikian JP. Bone mineral density in women with type II diabetes mellitus. J Bone Miner Res. 1989; 4:97–101.
13. Okuno Y, Nishizawa Y, Sekiya K, Hagiwara S, Miki T, Morii H. Total and regional bone mineral content in patients with non-insulin dependent diabetes mellitus. J Nutr Sci Vitaminol (Tokyo). 1991; 37:Suppl. S43–S49.
14. Wakasugi M, Wakao R, Tawata M, Gan N, Koizumi K, Onaya T. Bone mineral density measured by dual energy X-ray absorptiometry in patients with non-insulin-dependent diabetes mellitus. Bone. 1993; 14:29–33.
15. Sosa M, Dominguez M, Navarro MC, Segarra MC, Hernandez D, de Pablos P, Betancor P. Bone mineral metabolism is normal in non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1996; 10:201–205.
16. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992; 41:1422–1428.
17. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996; 382:250–252.
18. do Prado WL, de Piano A, Lazaretti-Castro M, de Mello MT, Stella SG, Tufik S, do Nascimento CM, Oyama LM, Lofrano MC, Tock L, Caranti DA, Damaso AR. Relationship between bone mineral density, leptin and insulin concentration in Brazilian obese adolescents. J Bone Miner Metab. 2009; 27:613–619.
19. Pasco JA, Henry MJ, Kotowicz MA, Collier GR, Ball MJ, Ugoni AM, Nicholson GC. Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab. 2001; 86:1884–1887.
20. Blain H, Vuillemin A, Guillemin F, Durant R, Hanesse B, de Talance N, Doucet B, Jeandel C. Serum leptin level is a predictor of bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2002; 87:1030–1035.
21. Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf). 2001; 55:341–347.
22. Jürimäe J, Jürimäe T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008; 26:618–623.
23. Filip R, Raszewski G. Bone mineral density and bone turnover in relation to serum leptin, alpha-ketoglutarate and sex steroids in overweight and obese postmenopausal women. Clin Endocrinol (Oxf). 2009; 70:214–220.
24. Goulding A, Taylor RW. Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int. 1998; 63:456–458.
25. Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008; 23:870–878.
26. Goldstone AP, Howard JK, Lord GM, Ghatei MA, Gardiner JV, Wang ZL, Wang RM, Girgis SI, Bailey CJ, Bloom SR. Leptin prevents the fall in plasma osteocalcin during starvation in male mice. Biochem Biophys Res Commun. 2002; 295:475–481.
27. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004; 351:987–997.
28. Johansson H, Oden A, Lerner UH, Jutberger H, Lorentzon M, Barrett-Connor E, Karlsson MK, Ljunggren O, Smith U, McCloskey E, Kanis JA, Ohlsson C, Mellstrom D. High serum adiponectin predicts incident fractures in elderly men: Osteoporotic Fractures in Men (MrOS) Sweden. J Bone Miner Res. 2012; 27:1390–1396.
29. Wang F, Wang PX, Wu XL, Dang SY, Chen Y, Ni YY, Gao LH, Lu SY, Kuang Y, Huang L, Fei J, Wang ZG, Pang XF. Deficiency of adiponectin protects against ovariectomy-induced osteoporosis in mice. PLoS One. 2013; 8:e68497.
30. Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, Wu XP, Liao EY. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005; 309:99–109.
31. Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005; 331:520–526.
32. Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology. 2008; 149:634–641.
33. Fogelholm GM, Sievanen HT, Kukkonen-Harjula TK, Pasanen ME. Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int. 2001; 12:199–206.
34. Hawkins J, Cifuentes M, Pleshko NL, Ambia-Sobhan H, Shapses SA. Energy restriction is associated with lower bone mineral density of the tibia and femur in lean but not obese female rats. J Nutr. 2010; 140:31–37.
35. Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004; 89:1196–1199.
36. Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985; 76:370–373.
37. Koh JM, Khang YH, Jung CH, Bae S, Kim DJ, Chung YE, Kim GS. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. 2005; 16:1263–1271.
38. Müller B. Cytokine imbalance in non-immunological chronic disease. Cytokine. 2002; 18:334–339.
39. Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukoc Biol. 2008; 84:882–892.
40. Fontana L. Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Exp Gerontol. 2009; 44:41–45.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download