Abstract
Background
Aneuploidy has been suggested as one of the major causes of cancer from the time of Boveri. In support of this notion, many studies have shown that cancer cells exhibit aneuploidy. However, there are evidences that do not support the aneuploidy hypothesis. We have previously reported that the spindle assembly checkpoint protein BubR1 is acetylated in mitosis and that the acetylation of BubR1 is crucial for checkpoint maintenance and chromosome-spindle attachment. Mice heterozygous for acetylation-deficient BubR1 (K243R/+) spontaneously develop cancer with chromosome instability. As K243R/+ mice develop hepatocellular carcinoma, we set out to test if chromosome mis-segregation was the cause of their liver cancer.
Methods
Primary hepatocytes in the regenerating liver after partial hepatectomy (PH) were analyzed and compared for various mitotic parameters.
Results
Primary hepatocytes isolated from K243R/+ mice after PH displayed a marked increase of chromosome misalignment, accompanied by an increase of micronuclei. In comparison, the number of nuclei per cell and the centrosome numbers were not different between wild-type and K243R/+ mice. Taken together, chromosome mis-segregation provokes tumorigenesis in mouse liver.
More than one hundred years ago, German biologist Theodor Boveri proposed that malignant tumors begin from a single cell in which the chromosomes are scrambled, resulting in uncontrolled cell growth [1,2,3]. His proposal led to the hypothesis that aneuploidy, an aberrant number of chrom osomes in a cell, is the cause of cancer. Indeed, a majority of cancers display aneuploidy. However, some data oppose the aneuploidy hypothesis; in yeast, aneuploidy causes proteotoxic stress and inhibition of cell growth [4,5] and in mouse, a certain level of aneuploidy is tumor suppressive due to growth inhibition. Aneuploidy becomes oncogenic only when combined with genotoxic stress [6,7]. Therefore, whether aneuploidy is the cause or the result of cancer is still at debate.
Aneuploidy can result from a compromised spindle assembly checkpoint (SAC), which monitors the bipolar spindle attachment to kinetochores. SAC ensures that the duplicated chromosomes do not divide into two cells until all chromosomes are properly attached to microtubule spindles [8,9,10]. We, and others, have shown previously that among the SAC components, BubR1 plays a critical role in SAC signaling; it is a critical component in the inhibition of APC/C and plays a pivotal role in the stable maintenance of kinetochore microtubule attachment in metaphase [11,12,13,14]. For BubR1's dual activity in mitosis, acetylation at lysine 250 (lysine 243 in mouse) is critical [15]. Further, we have shown that the acetylation requires the tumor suppressor BRCA2, which functions as a scaffold to bring the acetyl transferase P300/CBP-associated factor (PCAF) to mediate acetylation of BubR1 in prometaphase [16]. Mice heterozygous for acetylation-deficient BubR1 allele (K243R/+) develop spontaneous tumors due to chromosome mis-segregation, corroborating that BubR1 acetylation is indeed tumor suppressive [11]. From the mouse study, it was revealed that BubR1 acetylation is required to recruit the PP2A-B56α subunit to the kinetochore, counteracting the excessive Aurora B activity and maintaining stable chromosome- microtubule attachments. This study revealed that BubR1 acetylation has dual roles in mitosis: it is required for SAC maintenance and chromosome congression [11]. Therefore, spontaneous cancer development in K243R/+ mice is due to the marked increase of chromosome mis-segregation with failure in chromosome-spindle attachment and weakened SAC activity.
The liver is a terminally differentiated organ, thus cell division is rare. It has been known for some time that adult liver cells are composed of binuclear or tetranuclear cells, hence are polyploidy in karyotypes. Polyploidy in liver cells is due to the incompletion of cytokinesis [17]. As loss of BubR1 acetylation promotes spontaneous tumorigenesis, including in the liver [11], we asked how polyploidy in the liver contributes to tumorigenesis by analyzing regenerating hepatocytes in K243R/+ mice after partial hepatectomy (PH).
The generation of K243R/+ mice was previously reported [11]. All mice were housed in a specific pathogen-free facility or semiconventional (virus antibody-free) facility in Seoul National University. The Institutional Animal Care and Use Committee of Seoul National University (SNU-090630-3) approved the animal and the experimental protocols. We strictly followed the guidelines, policies, and regulations for the Care and Use of Laboratory Animals in Seoul National University.
Mice, 10 to 15 weeks old, were anesthetized using a mixture of isoflurane/oxygen. Seventy percent PH, which involves excision of the medial and left lateral lobes, was performed, according to Mitchell and Willenbring [18]. Three each of wild-type and K243R/+ mice were subjected to PH and liver biopsies were performed at each time point. After the procedure, the mice were kept ad libitum and sacrificed by cervical dislocation. Harvested livers were processed for subsequent analyses.
The statistical data were analyzed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). The Student t test was used for statistical analysis and the mean±standard error of the mean is shown as indicated. P values are marked.
Immunofluorescence and immunohistochemical assays of paraffin- embedded liver tissues were conducted with slight modifications from the previously described methods [19]. In brief, liver tissue specimens were collected after PH and embedded in paraffin. After deparaffinization, hydration, fixation, and antigen retrieval, paraffin sections were either stained with hematoxylin and eosin or the following primary antibodies. After blocking, specimens were stained with Ki-67 (1:500, Abcam, Cambridge, UK), γ-tubulin (T6557, T5192, 1:1,000, Sigma Aldrich, St. Louis, MO, USA), and Alexa Fluor 488 phalloidin (1:50, Invitrogen, Grand Island, NY, USA), followed by incubation with appropriate secondary antibodies.
Previously, we demonstrated that the presence of one acetylation- deficient BubR1 allele leads to robust tumorigenesis through failure of the SAC maintenance and chromosome congression [11]. Although mouse embryonic fibroblasts (MEFs) are a valuable resource from mice, they are comprised of a heterogeneous origin and are difficult to synchronize at a particular cell cycle stage.
To confirm the results in an in vivo system and to understand the molecular basis of liver tumorigenesis in K243R/+ mice, we set out to test the process of cell proliferation in liver regeneration after surgery. When two-third of the liver is hepatectomized, a large proportion of hepatocytes in G0 stage enter the proliferating cell cycle 1 to 2 days postoperation. These mitotic cells later differentiate and reconstitute the fully developed tissue [18].
Beginning at 24 hours posthepatectomy, we assessed and compared the proliferative capacity and integrity of mitosis in wild type (WT) and K243R/+ mice by analyzing the regenerating liver. For this, liver tissue specimens were collected in a time-course manner after hepatectomy. The biopsied liver tissues were embedded in paraffin, and finally analyzed with immunohistochemistry. The mice were euthanized for 72 hours during the experiment. The ability to enter the cell cycle was similar in the WT and K243R/+ hepatocytes of the regenerating livers (Fig. 1A, C). Seven days postoperation, the liver mass was restored in both genotypes without any significant difference. Concordantly, hepatocytes positive for Ki-67, which marks proliferating cells, accumulated to a similar degree (Fig. 1B, C). Interestingly, K243R/+ hepatocytes appeared to proliferate faster, compared to WT (Fig. 1).
When we assessed the chromosomes in mitotic cells, lagging chromosomes were frequently detected in the regenerating K243R/+ liver (Fig. 2A). Lagging chromosomes result from faulty attachment of microtubules to kinetochores, such as merotely, a form of microtubule attachment to a kinetochore from the opposite pole [20,21], and are thought to be the major source of aneuploidy. The result corroborates our previous finding from MEFs that having one allele of acetylation-deficient BubR1 precipitates aneuploidy [11]. It demonstrates that liver regeneration after PH reflects forced mitosis in vivo, and therefore, is a reliable system for assessing tumorigenesis in relation to proliferation.
In the paraffin-embedded liver sections of K243R/+ after PH, we were able to assess the congression, or metaphase chromosome alignment, as well. Consistent with the apparent lagging chromosomes, we detected a marked increase of congression defects in metaphase (Fig. 1C, 2B, left). Accordingly, chromosome mis-segregation, displayed by lagging chromosomes, and anaphase bridges were markedly increased in the regenerating hepatocytes of K243R/+ mice (Fig. 2B, right).
Abnormal centrosome numbers can result in multipolar segregation of chromosomes, resulting in chromosome instability. We asked whether centrosome numbers were affected in the regenerating livers of K243R/+ mice. Between wild-type and K243R/+ mice, there was not an apparent difference in centrosome numbers (Fig. 3). Therefore, the chromosome mis-segregation (Fig. 2) in K243R/+ mouse liver is not due to aberrant centrosome numbers.
A fully differentiated liver naturally harbors binuclear cells as a result of cytokinesis failure, thus polyploidy is not always a genetic insult in the liver [17]. However, tetraploidy has been suggested as a prerequisite for aneuploidy [22,23]. Therefore, we compared the incidence of mononuclear and binuclear cells in regeneration at postoperative day seven. Fifty-seven and forty high-power fields were observed from the livers of three K243R/+ and three WT mice, respectively. Our observations showed that there was no significant difference in the proportion of binuclear cells in K243R/+ livers during regeneration (Fig. 4A).
Finally, we asked whether there was significant increase of DNA damage in K243R/+ mice during liver regeneration. Immunostaining with an anti-γH2AX antibody and the TUNEL assay revealed that there was not a significant difference (data not shown).
As we reported, mice heterozygous for acetylation-deficient BubR1 precipitate mitotic infidelity, manifested by massive chromosome mis-segregation and micronuclei. Micronuclei were observed in regenerating hepatocytes as well, and the incidence was 5-fold higher in K243R/+ compared to wild-type [11]. In this report, we showed that mitotic infidelity in K243R/+ mice is seen in regenerating hepatocytes after PH. We showed that the polyploidy resulting from binuclear cells of the liver is not the prerequisite for aneuploidy in regenerating liver. Instead, the aneuploidy resulted from aberrant mitosis. Collectively, liver regeneration after PH confirmed that the partial impairment of BubR1 acetylation led to mitotic infidelities during proliferation, initiating tumorigenesis in mice (Fig. 4B). As liver regeneration after PH faithfully recapitulates the proliferation burden, our experiments can be applied to assessing the mitotic integrity in other mouse models.
Figures and Tables
Fig. 1
(A) Proliferation capacity in regenerating hepatocytes measured by counting the mitotic cells (mitotic index) or Ki-67-positive cells (B) at the indicated time points after partial hepatectomy (PH). The values in the graph are the mean of three independent experiments (mean±SEM). (C) The regenerating liver sections were stained with H&E (upper panel) and an antibody directed against Ki-67 (lower panel). Mitotic cells are marked in the brackets. Scale bar, 50 µm.
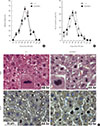
Fig. 2
(A) Chromosome misalignments (left) and lagging chromosomes (right) in regenerating K243R/+ hepatocytes. Scale bar, 10 µm. (B) The incidence of congression defects and lagging chromosomes compared with that of wild type (independent-samples t test) are represented as bar graphs.
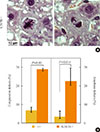
Fig. 3
Number of centrosomes in liver sections analyzed by anti-γ-tubulin, phalloidin, and diamidino-2-phenylindole immunostaining.
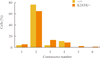
Fig. 4
(A) Comparison of the number of nuclei in the regenerating hepatocytes of WT and K243R/+ mice. The graph represents the analysis of hepatocytes from three of each WT and K243R/+ mice, subjected to partial hepatectomy (PH). The exact number of hepatocytes counted in each group is shown in the table. (B) Model showing how the PH experiment contributed to the understanding of the mouse hepatocellular carcinoma development with chromosome instability in K243R/+ mice.

ACKNOWLEDGMENTS
The Cancer Control Program of the Korean Ministry of Health (1220210) supported this work.
References
1. Boveri T. Ueber mehrpolige mitosen als mittel zur analyse des zellkerns. Vehr d phys med Ges zu Wurzburg N. 1902; 35:67–90.
2. Boveri T. Concerning the origin of malignant tumours by Theodor Boveri: translated and annotated by Henry Harris. J Cell Sci. 2008; 121:Suppl 1. 1–84.
3. Harris H. Concerning the origin of malignant tumours by Theodor Boveri: translated and annotated by Henry Harris: preface. J Cell Sci. 2008; 121:Suppl 1. v–vi.
4. Tang YC, Amon A. Gene copy-number alterations: a cost-benefit analysis. Cell. 2013; 152:394–405.
5. Oromendia AB, Dodgson SE, Amon A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 2012; 26:2696–2708.
6. Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol. 2009; 10:478–487.
7. Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2007; 11:25–36.
8. Funabiki H, Wynne DJ. Making an effective switch at the kinetochore by phosphorylation and dephosphorylation. Chromosoma. 2013; 122:135–158.
9. Logarinho E, Bousbaa H. Kinetochore-microtubule interactions "in check" by Bub1, Bub3 and BubR1: The dual task of attaching and signalling. Cell Cycle. 2008; 7:1763–1768.
10. Yu H. Regulation of APC-Cdc20 by the spindle checkpoint. Curr Opin Cell Biol. 2002; 14:706–714.
11. Park I, Lee HO, Choi E, Lee YK, Kwon MS, Min J, Park PG, Lee S, Kong YY, Gong G, Lee H. Loss of BubR1 acetylation causes defects in spindle assembly checkpoint signaling and promotes tumor formation. J Cell Biol. 2013; 202:295–309.
12. Xu P, Raetz EA, Kitagawa M, Virshup DM, Lee SH. BUBR1 recruits PP2A via the B56 family of targeting subunits to promote chromosome congression. Biol Open. 2013; 2:479–486.
13. Kruse T, Zhang G, Larsen MS, Lischetti T, Streicher W, Kragh Nielsen T, Bjorn SP, Nilsson J. Direct binding between BubR1 and B56-PP2A phosphatase complexes regulate mitotic progression. J Cell Sci. 2013; 126(Pt 5):1086–1092.
14. Suijkerbuijk SJ, Vleugel M, Teixeira A, Kops GJ. Integration of kinase and phosphatase activities by BUBR1 ensures formation of stable kinetochore-microtubule attachments. Dev Cell. 2012; 23:745–755.
15. Choi E, Choe H, Min J, Choi JY, Kim J, Lee H. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. EMBO J. 2009; 28:2077–2089.
16. Choi E, Park PG, Lee HO, Lee YK, Kang GH, Lee JW, Han W, Lee HC, Noh DY, Lekomtsev S, Lee H. BRCA2 fine-tunes the spindle assembly checkpoint through reinforcement of BubR1 acetylation. Dev Cell. 2012; 22:295–308.
17. Guidotti JE, Bregerie O, Robert A, Debey P, Brechot C, Desdouets C. Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J Biol Chem. 2003; 278:19095–19101.
18. Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008; 3:1167–1170.
19. Lee YK, Choi E, Kim MA, Park PG, Park NH, Lee H. BubR1 as a prognostic marker for recurrence-free survival rates in epithelial ovarian ca ncers. Br J Cancer. 2009; 101:504–510.
20. Thompson SL, Compton DA. Chromosome missegregation in human cells arises through specific types of kinetochore-microtubule attachment errors. Proc Natl Acad Sci U S A. 2011; 108:17974–17978.
21. Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol. 2001; 153:517–527.
22. Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007; 17:157–162.
23. Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004; 5:45–54.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download