See the reply "Response: Expression of Glucagon-Like Peptide-1 Receptor in Papillary Thyroid Carcinoma and Its Clinicopathologic Significance (Endocrinol Metab 2014;29:536-44, Min Jung Jung et al.)" in Volume 30 on page 233.
Abstract
Background
Incretin-based therapies are rapidly becoming one of the main glycemic control strategies in diabetes. Considering the large numbers of papillary thyroid carcinomas (PTCs) and possible effects of glucagon-like peptide-1 (GLP-1) on cell proliferation, the expression of GLP-1 receptor (GLP-1R) in PTC is likely to have clinical significance. We performed this study to evaluate the expression of GLP-1R in PTC and the clinical meaning of GLP-1R expression in PTC.
Methods
Fifty-six cases of PTC, four cases of medullary thyroid cancer (MTC), seven cases of nodular hyperplasia and 56 normal thyroid tissue samples were selected for immunostaining for GLP-1R. Clinical parameters were obtained by retrospective review of medical records.
Results
Immunohistochemical staining for GLP-1R showed immunoreactivity in 18 of 56 cases of PTC (32.1%). All four cases of MTC exhibited cytoplasmic GLP-1R expression. Nodular hyperplasia exhibited immunoreactivity in two of seven cases (28.6%). All normal thyroid follicular cells showed negative immunoreactivity. In univariable and multivariable analyses, tumor multifocality was negatively correlated with GLP-1R expression. Extrathyroidal extension showed positive association with GLP-1R expression that was almost significant. Sex, age, tumor size, and lymph node metastasis were not significantly associated with GLP-1R expression.
Conclusion
Some parts of PTC tissues express GLP-1R, and GLP-1R expression in PTC was negatively correlated with tumor multifocality. The long-term influence of pharmacologically increased GLP-1 on thyroid follicular cells and development and progression of tumors originating from thyroid follicular cells should be investigated.
Incretin-based therapy is rapidly becoming a main glycemic control strategy in diabetes [1,2]. With heterogeneous evidence of the cardiovascular benefit, mortality, and potential risks of intensive glycemic control reported in several recent clinical trials, achieving good blood glucose control without increasing cardiovascular risk factors such as hypoglycemia and body weight gain is becoming more important [3,4,5,6,7]. With this in mind, incretin, especially glucagon-like peptide-1 (GLP-1)-based therapy, which includes GLP-1 receptor (GLP-1R) agonist and dipeptidyl peptidase-4 (DPP-4) inhibitor treatment, has several benefits. It increases insulin secretion in a glucose-dependent manner, does not cause hypoglycemia and does not cause body weight gain [8]. Despite such benefits, concerns about pancreatitis, pancreatic ductal hyperplasia, pancreatic cancer and thyroid cancer remain in long-term incretin-based treatment due to the effects of GLP-1 on cell growth and proliferation [9,10,11,12,13]. According to several large recent clinical trials and thorough examination of GLP-1R expression in various pancreatic pathologic conditions, these kinds of concerns in humans have decreased but have not clearly vanished [14,15,16,17]. The association of incretin-based therapy with thyroid cancer originates from preclinical data showing the elevation of calcitonin and occurrence of hyperplasia and tumors arising from rat thyroid gland parafollicular C cells after treatment with liraglutide, a GLP-1R agonist [18,19,20]. Unlike the rat thyroid gland, the human thyroid gland has far fewer C cells and no case of medullary thyroid cancer (MTC) has been reported before now [21,22]. However, some data suggest an increased risk of thyroid cancer in patients treated with a GLP-1R agonist or DPP-4 inhibitor, although the methods of analysis were questioned after publication [13]. Moreover Gier et al. [22] recently reported the possibility of GLP-1R expression in papillary thyroid carcinoma (PTC). Considering the increasing numbers of PTC cases, the expression of GLP-1R in a subset of PTCs is likely to be more significant than the possibility of C cell neoplasms. We performed this study to confirm the existence of GLP-1R in PTC tissue and the clinical meaning of GLP-1R expression in PTC.
Individuals were selected for the present study from the Department of Pathology at Kosin University Gospel Hospital. A database was searched for cases submitted for postoperative pathologic diagnosis between March 2013 and September 2014. Fifty-six cases of PTC, four cases of MTC, and seven cases of nodular hyperplasia were selected for immunostaining and analysis of clinical parameters. Peritumoral normal thyroid tissues from 56 PTC patients were used as a normal thyroid tissue control. Clinical parameters were obtained by retrospective review of medical records for the selected cases.
Use of all slides, paraffin-embedded tissue blocks and clinical information was ethically approved by the Institutional Review Board (KUGH no. 91961-ABG-14-032).
Immunohistochemical staining was carried out on representative formalin-fixed, paraffin-embedded tissues using the Bond Polymer Refine Detection system (Leica Biosystems Newcastle Ltd., Newcastle, UK) according to the manufacturer's instructions, with minor modifications. Briefly, 4-µm sections of formalin-fixed and paraffin-embedded tissues were deparaffinized using Bond Dewax Solution (Leica Biosystems Newcastle Ltd.) and antigen retrieval was achieved by incubation with Bond Epitope Retrieval Solution 1 (Leica Biosystems Newcastle Ltd.) for 20 minutes at 98℃. Then, endogenous peroxidase was quenched by incubation with hydrogen peroxide for 15 minutes. Sections were incubated for 15 minutes at ambient temperate with a polyclonal rabbit anti-human GLP-1R antibody (ab39072, Abcam, Cambridge, UK) at a dilution of 1:1,000, using a biotin-free polymeric horseradish peroxidase linker antibody conjugate system. The signal was developed with the chromogen 3,3'-diaminobenzidine in a Bond-Max automatic slide stainer (Leica Biosystems Melbourne Pty Ltd., Melbourne, Australia). For comparison, four cases of MTC and seven cases of nodular hyperplasia diagnosed during the same period were immunostained for GLP-1R. Islet cells of the pancreas and thyroid vessels were used as positive and negative control tissues for immunostaining, respectively.
Whole tumors were evaluated for staining intensity and distribution of stained cells. Staining intensity was scored as follows: absent (no staining or ambiguous staining; Fig. 1A, B), weak (weaker staining than in control tissue; Fig. 1C), or strong (similar staining intensity as for control tissue; Fig. 1D). The distribution of stained cells was evaluated as the average percentage of stained tumor cells. A strong intensity was considered positive, regardless of the distribution. A weak intensity was considered positive when the stained cells accounted for more than 5% of the whole tumor section. Tumors exhibiting weak immunoreactivity in less than 5% of the whole tumor were considered negative (ambiguous staining).
We retrospectively reviewed medical records for the patients who were selected for GLP-1R immunohistochemical staining. Age, sex, and serum levels of thyroid hormones, thyroglobulin, anti-thyroglobulin antibody and calcitonin, checked before surgery, were reviewed. Tumor size, lymph node (LN) metastasis status, extrathyroidal extension (ETE) and tumor multifocality were reviewed by a pathologist postoperatively with resected tumor tissues. For analysis of the correlations of GLP-1R positivity with clinicopathologic parameters in PTC, age and tumor size were dichotomized as <45 and ≥45 years and <1 and ≥1 cm, respectively.
Statistical significance for continuous variables was assessed by Student t test. Univariable analysis of the significance of GLP-1R expression was performed by chi-square test and Fisher exact test; multivariable analysis of the significance of GLP-1R expression was conducted by logistic regression. P values of <0.05 were considered to indicate statistical significance. Data were analyzed using SPSS version 18.0 (IBM Co., Armonk, NY, USA).
Immunohistochemical staining of GLP-1R in PTC showed cytoplasmic expression in 18 of 56 cases (32.1%) (Table 1). The expression of GLP-1R was strong in two cases (100% and 80% intensity) and weak in 16 cases, in which the distribution was usually diffuse and over 50%, except for two cases with distributions of 20%. All four cases of MTC exhibited strong cytoplasmic expression of GLP-1R, albeit the staining was focal (Table 1, Fig. 2A, B). The mean serum calcitonin level was 1.65±1.78 in the PTC group and 629.3±314.7 pg/mL in the MTC group. There was strong cytoplasmic expression of GLP-1R in two of seven cases of nodular hyperplasia (28.6%) (Table 1, Fig. 2C, D). Very few non-neoplastic follicular cells in the adjacent thyroid parenchyma were positive for GLP-1R (less than 5% of the distribution) or exhibited ambiguous granules in contact with colloid showing non-specific immunoreactivity (Fig. 3). These findings were insufficient to interpret as positive immunoreactivity. Some cases showed strong GLP-1R expression in C cells of the adjacent thyroid parenchyma (Fig. 3A).
There were no significant differences in age, sex ratio, or tumor size between GLP-1-positive and -negative PTC (Table 2). Mean thyroglobulin was 36.77±59.9 ng/mL (range, 0.89 to 311.00) in the GLP-1R-negative group and 38.56±58.6 ng/mL (range, 3.33 to 255.80) in the GLP-1R-positive group (P=0.916); serum calcitonin was 1.634±1.917 pg/mL (range, 1.0 to 11.8) in the GLP-1R-negative group and 1.676±1.476 pg/mL (range, 1.0 to 5.5) in the GLP-1R-positive group (P=0.974). Preoperative levels of thyroid hormones, including total triiodothyronine, free thyroxine, and thyroid-stimulating hormone, were similar in the two groups.
We compared several clinical parameters, including age, sex, and tumor size, and pathologic prognostic parameters such as LN metastasis, multifocality, ETE of tumors and anti-thyroglobulin antibody status between the GLP-1R-positive and -negative groups (Table 3). In univariable analyses, tumor multifocality was significantly negatively correlated with expression of GLP-1R in PTC (odds ratio [OR], 0.257; 95% confidence interval [CI], 0.071 to 0.926; P=0.044), and extrathyroidal tumor extension showed a non-significant positive correlation with GLP-1R expression (OR, 3.022; 95% CI, 0.947 to 9.646; P=0.057). In multivariable analyses, tumor multifocality was the only variable that was negatively correlated with expression of GLP-1R in PTC (adjusted OR, 0.172; 95% CI, 0.037 to 0.797; P=0.024); extrathyroidal tumor extension was non-significantly associated with GLP-1R expression (OR, 3.46; 95% CI, 0.879 to 13.618; P=0.076). Sex, patient age (<45 or ≥45 years), tumor size (<1 or ≥1 cm), LN metastasis, and presence of anti-thyroglobulin antibodies were not significantly associated with GLP-1R expression.
Our objectives in this study were to determine the expression of GLP-1R in PTC and to evaluate the clinicopathologic significance of GLP-1R expression in PTC. This study demonstrated that some PTC tissues expressed GLP-1R (18 of 56 cases, 32.1%). There are few data on the expression of GLP-1R in PTC and normal thyroid tissues. Gier et al. [22] reported positive immunoreactivity for GLP-1R in PTC tissues, detected using a polyclonal anti-GLP-1R antibody. Their study aimed to establish whether C cells in human MTC, C cell hyperplasia and normal human thyroid express GLP-1R. The expression of GLP-1R in PTC was an unexpected finding and only three of the 17 PTC tissue samples (18%) expressed GLP-1R. The number of PTC tissues in our study was higher than in previous studies, reducing the possibility of negative or positive immunoreactivity occurring by chance. In our study, we ascertained the expression of GLP-1R in PTC, as well as the rate of GLP-1R expression in PTC which reached almost 30%, more than in previous reports. Recently, Waser et al. [17] reported no expression of GLP-1R in eight cases of PTC by immunohistochemical staining using an anti-GLP-1R monoclonal antibody (MAb 3F52) and in vitro autoradiography. They suggested that the discrepancy compared with previous reports was caused by the different specificity of the antibody used. Therefore, thorough examination of GLP-1R expression with different specific antibodies and various validation methods in large numbers of PTC samples is warranted.
Interestingly, we found GLP-1R immunoreactivity in two of seven cases of nodular hyperplasia (28.6%), but none of the 56 normal peritumoral thyroid tissue samples showed GLP-1R expression. Regarding the expression of GLP-1R in non-tumorous tissues, our result concurs with several previous studies. Waser et al. [21] examined human tissues including normal and cancerous tissues and found that normal human thyroid tissue from non-neoplastic and neoplastic thyroid glands did not express GLP-1R (0/6, 0%). Korner et al. [23] studied the expression of GLP-1R in various human thyroid tissues by scintigraphy. They demonstrated that few normal thyroid tissue expressed GLP-1R (1/18, 6%). Gier et al. [22] reported different expression of GLP-1R in non-tumorous thyroid tissue according to inflammation status. Normal thyroid tissues with inflammation expressed GLP-1R (2/3, 33%), but normal thyroid tissue without inflammation did not show GLP-1R expression (0/7, 0%). In our study, we did not distinguish between non-tumorous peritumoral thyroid tissue according to inflammation, but none of the tissue samples showed GLP-1R expression according to our definition of positive immunoreactivity for GLP-1R expression. However, taken together, the negative GLP-1R expression in normal thyroid tissues, positive GLP-1R immunoreactivity in non-tumorous thyroid tissues with inflammation and positive GLP-1R immunoreactivity in benign and malignant tumorous conditions suggest that GLP-1R may be aberrantly induced in cells originating from thyroid follicles by inflammation, cellular hyperplasia or tumorigenesis. The pathogenic mechanism is not known and the GLP-1R ligand GLP-1 may affect the development or behavior of the tumor.
In this study, we analyzed several clinicopathologic factors known to be associated with the prognosis in PTC, including age, gender, primary tumor size, extrathyroidal tumor extension, LN metastasis, and tumor multifocality [24,25,26,27]. Among these factors, only tumor multifocality was significantly negatively correlated with GLP-1R expression in both univariable and multivariable analyses. This finding suggests that GLP-1R expression is associated with less possibility of multifocality in PTC and can be considered a good prognostic signal, although the prognostic significance of multifocality in PTC is still controversial. Several studies suggest that multifocal PTC was associated with an increased risk of regional LN metastasis or tumor recurrence [27,28,29]. However, other studies did not support the clinical significance of tumor multifocality [30,31,32]. Moreover, although statistically insignificant, extrathyroidal tumor extension, which is known to be associated with poor prognosis in PTC, showed a non-significant positive correlation with GLP-1R expression in PTC in our study. Until now, there has been no report on the clinical significance of GLP-1R expression in PTC. Therefore, the prognostic significance of GLP-1R expression in PTC remains unclear.
Although there are no data for the effects of GLP-1R activation on the proliferation and apoptosis of thyroid cancer cells originating from follicular cells, we can deduce the pathogenic mechanism of clinical significance from several previous reports. The effects of GLP-1R activation are mediated by the activation of downstream signals that increase cellular cyclic AMP (cAMP) levels [33,34,35]. cAMP has both pro-apoptotic and anti-apoptotic action in a large number of different cells and even in the same cell. Whether cAMP activates or inhibits apoptosis in a particular cell is determined by the balance between a protein kinase A-mediated pro-apoptotic response and an exchange protein activated by cAMP (Epac)-mediated anti-apoptotic response [36]. Actually, several previous studies have shown that GLP-1 has different effects on proliferation and apoptosis in different cell types. In pancreatic β-cells and thyroid C cells, GLP-1 can activate proliferation and inhibit apoptosis [37,38]. By contrast, in CT26 murine colon cancer cell line, GLP-1R agonist treatment inhibited cellular proliferation and induced apoptosis [39].
This study had several limitations. First, we used a commercial GLP-1R polyclonal antibody and did not validate its specificity. Second, the number of cases in this study was too small to determine the clinicopathologic significance of GLP-1R expression in PTC. Finally, we only used immunohistochemical staining to demonstrate the expression of GLP-1R in thyroid tissue. Nevertheless, this is the first study to reveal GLP-1R expression in a large number of PTC tissue samples. Also, this is the first study to evaluate the clinicopathologic meaning of GLP-1R expression in PTC.
In conclusion, some parts of PTC tissues express GLP-1R and GLP-1R expression in PTC was negatively correlated with tumor multifocality. Further study of the effect of GLP-1 on thyroid follicular cells and longitudinal observation of the association of long-term GLP-1R activation with PTC is needed to clarify the meaning of GLP-1R expression in PTC and the long-term effects of incretin-based therapy on the thyroid.
Figures and Tables
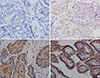 | Fig. 1Glucagon-like peptide-1 receptor (GLP-1R) expression in papillary thyroid carcinoma (PTC) samples stained by immunohistochemistry. (A) No immunoreactivity (×400). (B) Ambiguous immunoreactivity. (×400) No and ambiguous immunoreactivity were considered as negative GLP-1R expression. (C) Weak immunoreactivity (×400). (D) Strong immunoreactivity (×400). (C) and (D) demonstrate positive GLP-1R expression in the cytoplasm of PTC cells and were considered as positive GLP-1R expression. |
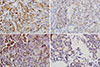 | Fig. 2Glucagon-like peptide-1 receptor (GLP-1R) expression in medullary thyroid carcinoma and nodular hyperplasia. (A) and (B) show medullary thyroid carcinoma samples positive for GLP-1R, with strong (A) and mixed strong and weak (×400) (B) immunoreactivity (×400). (C) and (D) show nodular hyperplasia samples positive for GLP-1R. (C) Strong immunoreactivity (×400). (D) Weak immunoreactivity (×400). |
 | Fig. 3Glucagon-like peptide-1 receptor (GLP-1R) expression in very few non-neoplastic follicular and C cells was considered as negative immunoreactivity. (A) Focal immunoreactivity (<5%) for GLP-1R in non-neoplastic follicular cells and C cells (×400). (B) Non-specific ambiguous granular immunoreactivity in a few follicular cells in contact with colloid, which was considered as negative immunoreactivity (×400). |
References
1. American Diabetes Association. Standards of medical care in diabetes: 2014. Diabetes Care. 2014; 37:Suppl 1. S14–S80.
2. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez G, Davidson MH. American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013; 19:327–336.
3. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577–1589.
4. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005; 353:2643–2653.
5. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
6. ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–2572.
7. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129–139.
8. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006; 368:1696–1705.
9. Denker PS, Dimarco PE. Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care. 2006; 29:471.
10. Cure P, Pileggi A, Alejandro R. Exenatide and rare adverse events. N Engl J Med. 2008; 358:1969–1970.
11. Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009; 374:39–47.
12. Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000; 141:4600–4605.
13. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011; 141:150–156.
14. Hegedus L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab. 2011; 96:853–860.
15. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013; 369:1317–1326.
16. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013; 369:1327–1335.
17. Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-like-peptide-1 receptor expression in normal and diseased human thyroid and pancreas. Mod Pathol. 2014; 09. 12. Epub. DOI: http://dx.doi.org/10.1038/modpathol.2014.113.
18. Crespel A, De Boisvilliers F, Gros L, Kervran A. Effects of glucagon and glucagon-like peptide-1-(7-36) amide on C cells from rat thyroid and medullary thyroid carcinoma CA-77 cell line. Endocrinology. 1996; 137:3674–3680.
19. Lamari Y, Boissard C, Moukhtar MS, Jullienne A, Rosselin G, Garel JM. Expression of glucagon-like peptide 1 receptor in a murine C cell line: regulation of calcitonin gene by glucagon-like peptide 1. FEBS Lett. 1996; 393:248–252.
20. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010; 151:1473–1486.
21. Waser B, Beetschen K, Pellegata NS, Reubi JC. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology. 2011; 94:291–301.
22. Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab. 2012; 97:121–131.
23. Korner M, Stockli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007; 48:736–743.
24. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.
25. Schlumberger M, Pacini F, Wiersinga WM, Toft A, Smit JW, Sanchez Franco F, Lind P, Limbert E, Jarzab B, Jamar F, Duntas L, Cohen O, Berg G. Follow-up and management of differentiated thyroid carcinoma: a European perspective in clinical practice. Eur J Endocrinol. 2004; 151:539–548.
26. Cross S, Wei JP, Kim S, Brams DM. Selective surgery and adjuvant therapy based on risk classifications of well-differentiated thyroid cancer. J Surg Oncol. 2006; 94:678–682.
27. Ricci JA, Alfonso AE. Multifocal micropapillary thyroid cancer: a new indication for total thyroidectomy? Am Surg. 2012; 78:1211–1214.
28. Kim HJ, Sohn SY, Jang HW, Kim SW, Chung JH. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg. 2013; 37:376–384.
29. Zhao Q, Ming J, Liu C, Shi L, Xu X, Nie X, Huang T. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol. 2013; 20:746–752.
30. Schindler AM, van Melle G, Evequoz B, Scazziga B. Prognostic factors in papillary carcinoma of the thyroid. Cancer. 1991; 68:324–330.
31. Ross DS, Litofsky D, Ain KB, Bigos T, Brierley JD, Cooper DS, Haugen BR, Jonklaas J, Ladenson PW, Magner J, Robbins J, Skarulis MC, Steward DL, Maxon HR, Sherman SI. Recurrence after treatment of micropapillary thyroid cancer. Thyroid. 2009; 19:1043–1048.
32. Kim KJ, Kim SM, Lee YS, Chung WY, Chang HS, Park CS. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol. 2015; 22:125–131.
33. Dalle S, Burcelin R, Gourdy P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic beta-cell impairments in type 2 diabetes. Cell Signal. 2013; 25:570–579.
34. Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P. Glucagon-like peptide 1 (7-36) amide stimulates exocytosis in human pancreatic beta-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes. 1998; 47:57–65.
35. Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2'-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem. 2003; 278:8279–8285.
36. Insel PA, Zhang L, Murray F, Yokouchi H, Zambon AC. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol (Oxf). 2012; 204:277–287.
37. Madsen LW, Knauf JA, Gotfredsen C, Pilling A, Sjogren I, Andersen S, Andersen L, de Boer AS, Manova K, Barlas A, Vundavalli S, Nyborg NC, Knudsen LB, Moelck AM, Fagin JA. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012; 153:1538–1547.
38. Brubaker PL, Drucker DJ. Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004; 145:2653–2659.
39. Koehler JA, Kain T, Drucker DJ. Glucagon-like peptide-1 receptor activation inhibits growth and augments apoptosis in murine CT26 colon cancer cells. Endocrinology. 2011; 152:3362–3372.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download