This article has been corrected. See "Erratum: Correction of Figure. Clinical Implications of Various Criteria for the Biochemical Diagnosis of Insulinoma" in Volume 32 on page 306.
Abstract
Background
Among the various diagnostic criteria for insulinoma, the ratio criteria have been controversial. However, the amended insulin-glucose ratio exhibited excellent diagnostic performance in a recent retrospective cohort study, although it has not yet been validated in other patient cohorts. We examined the diagnostic performance of the current criteria of the Endocrine Society, insulin-glucose ratio, C-peptide-glucose ratio, and amended ratios in terms of differentiating insulinomas.
Methods
We reviewed the medical records of patients who underwent evaluation for hypoglycemia from 2000 to 2013. Fourteen patients with histopathologically confirmed insulinoma and 18 patients without clinical evidence of insulinoma were included. The results of a prolonged fast test were analyzed according to the abovementioned criteria.
Results
Fulfilling all three Endocrine Society criteria-plasma levels of glucose (<3.0 mmol/L), insulin (≥8 pmol/L), and C-peptide (≥0.2 nmol/L)-exhibited 100% sensitivity and 89% specificity. Fulfilling the glucose and C-peptide criteria showed 100% sensitivity and 83% specificity, while fulfilling the glucose and insulin criteria showed 100% sensitivity and 72% specificity. Among the ratio criteria, the insulin-glucose ratio [>24.0 (pmol/L)/(mmol/L)] gave the highest area under the receiver operating characteristic curve, with 93% sensitivity and 94% specificity.
Although insulinoma is a rare endocrine disease, it is the most common cause of hypoglycemia in apparently healthy adults if factitious hypoglycemia is excluded [1]. Because insulinoma occurs in the pancreas in the majority of patients, the pancreas is the first place where it is sought [2]. For this purpose, computed tomography (CT), magnetic resonance imaging, or endoscopic ultrasonography is performed, with insulinoma detection rates of approximately 70% to 80%, 85%, and 90%, respectively [1,3]. Even when insulinoma is not detected by imaging studies, the pancreas can be surgically explored if there is a strong clinical suspicion of insulinoma, or an invasive study, such as selective pancreatic arterial calcium injection with hepatic venous sampling, can be performed to identify pancreatogenous hypoglycemia before surgical exploration [2]. It is, therefore, of paramount importance to diagnose insulinoma with biochemical methods.
The documentation of endogenous hyperinsulinemic hypoglycemia is essential for the biochemical diagnosis of insulinoma. A prolonged fast test of up to 72 hours is considered the standard test to document this condition when a spontaneous hypoglycemic event is not observed [4]. The Endocrine Society Clinical Practice Guidelines (ESCPG) for adult hypoglycemic disorders define endogenous hyperinsulinemic hypoglycemia as symptoms and signs of hypoglycemia with a plasma glucose level of <3.0 mmol/L (55 mg/dL), an insulin level of ≥18 pmol/L (3.0 µU/mL), a C-peptide level of ≥0.2 nmol/L (0.6 ng/mL), and a proinsulin level of ≥5.0 pmol/L [1]. In addition, a glucagon stimulation test is recommended to reveal preservation of hepatic glycogen stores, which is mediated by the action of excess insulin or IGF-1 [1]. However, this definition has been criticized for not being clear regarding how many of these criteria should be met to confirm the diagnosis [5]. Each criterion of the ESCPG showed consistently high sensitivity of 90% to 100% but variable specificity in different studies [4,5,6]. Three recent studies that analyzed the diagnostic performance of the current criteria in cohorts including both patients and controls reported that the plasma insulin criteria had specificities of 15%, 19%, and 95% [4,5,6]. The diagnostic performance varies among populations and insulin and C-peptide assays. The ESCPG also stated that the insulin-glucose ratio has no diagnostic value for differentiating endogenous hyperinsulinism [1]. However, some experts still endorse use of the insulin-glucose ratio for diagnosis of insulinoma, because it reflects the effect of glucose concentration on insulin secretion [5,7,8].
Intriguingly, a retrospective German study reported that the amended insulin-glucose ratio exhibited excellent diagnostic value, with 98% sensitivity and 98% specificity, in insulinomas in 114 subjects who underwent the prolonged fast test for the evaluation of hypoglycemia [5]. The amended insulin-glucose ratio is the ratio of the plasma insulin concentration to the plasma glucose concentration minus 1.7 mmol/L. The subtraction of 1.7 mmol/L is based on the assumption that a negligible amount of insulin is secreted at plasma glucose concentrations below 1.7 mmol/L [9]. This ratio has a negative or infinite value when the plasma glucose level is below or exactly 1.7 mmol/L. In these cases, in the German study [5], a value of 100 was assigned for the amended insulin-glucose ratio (personal communication with Nauck MA). However, the diagnostic accuracy of the amended insulin-glucose ratio has not yet been validated in other patient cohorts. To address this, we evaluated the diagnostic performance of the current biochemical criteria of the ESCPG, insulin-glucose ratio, C-peptide-glucose ratio, amended insulin-glucose ratio, and amended C-peptide-glucose ratio for insulinomas.
We reviewed the medical records of all consecutive patients who did not have diabetes but who presented with hypoglycemia at Seoul National University Hospital from January 1, 2000 to January 31, 2013. Patients with results for the prolonged fast test were identified and included in the analysis. The insulinoma group was defined as patients with histopathologically confirmed insulinomas. The noninsulinoma group was defined as patients who had no clinical evidence of insulinoma. No patient had abnormal liver or renal function. This study protocol was approved by the Institutional Review Board at Seoul National University Hospital (registration number: 1307-113-506).
Patients fasted under the supervision of health care professionals. Capillary plasma glucose level was measured with a glucometer (OneTouch SureStep Hospital Meter, LifeScan, Milpitas, CA, USA) every 2 hours. When hypoglycemic symptoms developed or the capillary plasma glucose levels decreased to 3.3 mmol/L, we checked the capillary plasma glucose level on an hourly basis. The fasting test was terminated when one of the following conditions was met: (1) hypoglycemic symptoms were present and the capillary plasma glucose level was <2.5 mmol/L; (2) intolerable hypoglycemic symptoms occurred; (3) no hypoglycemic symptoms were present, but the capillary plasma glucose level was <2.5 mmol/L and the plasma glucose level was checked immediately and verified to be <2.5 mmol/L; and (4) 72 hours had elapsed since the last meal. At the time of discontinuation, venous blood was drawn for measurement of plasma glucose, insulin, and C-peptide levels. The capillary blood glucose level was only used to assist the decision to discontinue the test. Plasma glucose level, measured by laboratory determination, was used to differentiate insulinomas.
Plasma glucose level was measured by the glucose oxidase method (UniCel Dxc880i Synchron, Beckman Coulter, CA, USA). Plasma insulin and C-peptide levels were measured using commercial immunoradiometric assays. The insulin assays were provided by DIAsource (Brussels, Belgium; formerly BioSource) for 26 patients from January 2000 to April 2010, TFB (Tokyo, Japan) for five patients from April 2010 to April 2012, and IZOTOP (Budapest, Hungary) for one patient from April 2012 to January 2013. The detection limit was 7.4 pmol/L (1 µU/mL) for the DIAsource assay and 3.6 pmol/L (0.6 µU/mL) for the IZOTOP assay. Cross-reactivity with human proinsulin was 0.3% for the DIAsource assay, 0.3% for the TFB assay, and 40.0% for the IZOTOP assay. The C-peptide assays were provided by TFB (Tokyo, Japan) for 26 patients from January 2000 to April 2010 and IZOTOP (Budapest, Hungary) and for six patients from April 2010 to January 2013. The detection limit was 0.033 nmol/L (0.1 ng/mL) for the TFB assay and 0.0332 nmol/L (0.105 ng/mL) for the IZOTOP assay. Cross-reactivity with human proinsulin was 25.4% for the TFB assay and 27.1% for the IZOTOP assay.
The insulin, C-peptide, and glucose levels at the end of the prolonged fast test were used for the biochemical diagnosis of insulinoma. The ESCPG criteria were applied, and diagnostic performance was analyzed for each component of the criteria and by determining the number of criteria met. We measured the diagnostic performance of each ratio based on the cut off values from previous studies and also based on new cutoff values derived from our current data (those with the highest Youden index [sensitivity+specificity-1]). The cutoff values from previous studies were 32.2 (pmol/L)/(mmol/L) for the insulin-glucose ratio [5,7,8], 53.6 (pmol/L)/(mmol/L) peptide-glucose ratio, and 0.61 (nmol/L)/(mmol/L) for the amended C-peptide-glucose ratio [5]. The amended insulin-glucose ratio is insulin (pmol/L)/[glucose (mmol/L)-1.7 nmol/L] and the amended C-peptide-glucose ratio is C-peptide (nmol/L)/[glucose (mmol/L)-1.7 nmol/L]. When the plasma glucose level was ≤1.7 mmol/L, we imputed 0.1 mmol/L for the denominator of the amended ratios to avoid generation of a negative or infinite value.
We present the results for continuous variables as means and standard deviations or 95% confidence intervals. Fisher exact test and the Mann-Whitney U test were used to compare the two groups. Area under the receiver operating characteristic (ROC) curve, sensitivity, specificity, positive predictive value, and negative predictive value were calculated for each diagnostic criterion. Statistical analysis was performed using SPSS version 18.0 (IBM Co., Armonk, NY, USA) and R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Thirty-nine patients who presented with hypoglycemia during the study period underwent the prolonged fast test. Of these patients, seven were excluded for the following reasons: no follow-up data (n=2), insulin autoantibody syndrome (n=1), and no available insulin data (n=4). Fourteen patients with histopathologically confirmed insulinoma and eighteen patients with no clinical evidence of insulinoma during a mean follow-up of 47.4±43.0 months were finally included.
The mean size of the tumors was 1.6±0.5 cm. Tumors were located at the head (n=7), body (n=3), and tail (n=4) of the pancreas. Preoperative localization was done with CT scans at the time of presentation (n=12), a follow-up CT scan after 1 year (n=1), or selective pancreatic arterial calcium injection with hepatic venous sampling (n=1).
In the noninsulinoma group, six patients were diagnosed with reactive hypoglycemia after a mixed meal test; two patients were diagnosed with factitious hypoglycemia caused by erroneously taken sulfonylurea. In two patients, hypoglycemia was associated with either a herbal medication or an unknown diet pill. In the remaining eight patients, the possible cause of hypoglycemia was not identified.
Compared to the noninsulinoma group, BMI was significantly higher (26.7±3.4 kg/m2 vs. 22.7±4.2 kg/m2, P=0.003) and hemoglobin A1c was significantly lower (4.8%±0.2% vs. 5.7%±0.5%, P<0.001) in the insulinoma group. The proportion of patients who presented with neuroglycopenic symptoms such as loss of consciousness, seizure, and confusion was not significantly different between the insulinoma group and the non-insulinoma group (78% vs. 56%, P=0.175) (Table 1).
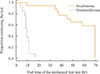
Most of the insulinoma patients (n=13, 93%) discontinued the prolonged fast test because their glucose level was below 2.5 mmol/L, whereas most of the noninsulinoma patients (n=11, 61%) discontinued after 72 hours had elapsed (Table 1). All of the insulinoma patients met the criteria for discontinuation of the test within 24 hours (range, 10 to 17), but only one noninsulinoma patient ended the test within 24 hours (Fig. 1).
We compared the plasma insulin, C-peptide, and glucose levels at the end of the prolonged fast test. The mean plasma glucose level was significantly lower in the insulinoma group than in the noninsulinoma group (2.07±0.58 mmol/L vs. 3.34±0.89 mmol/L, P<0.001). The mean insulin and C-peptide levels were significantly higher in the insulinoma group than in the non-insulinoma group (123.3±79.9 pmol/L vs. 39.6±17.6 pmol/L, P<0.001 for insulin; 1.07±0.48 nmol/L vs. 0.28±0.35 nmol/L, P<0.001 for C-peptide) (Table 1). However, the insulinoma and noninsulinoma groups showed considerable overlap for each value (Fig. 2). All of the insulinoma patients met the plasma glucose criterion (≤3.0 mmol/L) and six noninsulinoma patients (33%) also met the plasma glucose criterion (Table 2).
On the basis of the ESCPG criteria, fulfilling the criteria for glucose, insulin, and C-peptide exhibited a sensitivity of 100% (95% confidence interval [CI], 73 to 100) and a specificity of 89% (95% CI, 64 to 98) (Table 2). Fulfilling the glucose and C-peptide criteria showed a sensitivity of 100% (95% CI, 73 to 100) and a specificity of 83% (95% CI, 58 to 96); fulfilling the glucose and insulin criteria showed a sensitivity of 100% (95% CI, 73 to 100) and a specificity of 72% (95% CI, 46 to 89).
Among the ratio criteria, the insulin-glucose ratio demonstrated the highest area under the ROC curve (0.968; 95% CI, 0.918 to 1.00), although the difference compared to other ratio criteria was not statistically significant (Table 3) [10]. The cut-off value for insulin-glucose ratio with the highest Youden index was 24.0 (pmol/L)/(mmol/L), lower than that in a previous study [5]. Using this cutoff value, the sensitivity of the insulin-glucose ratio was 93% (95% CI, 64 to 100) and the specificity was 94% (95% CI, 71 to 100). Although the insulin-glucose ratio showed the highest area under the ROC curve, other ratio criteria exhibited comparable sensitivity and specificity (Table 2).
In this study, we examined the diagnostic performances of various biochemical criteria for insulinomas. Among them, fulfilling the glucose, insulin, and C-peptide criteria of the ESCPG demonstrated excellent sensitivity and specificity in differentiating insulinomas from other causes of spontaneous hypoglycemia. Compared to simultaneously fulfilling the glucose, insulin, and C-peptide criteria of the ESCPG, each component of the ESCPG criteria, combined glucose and insulin criteria, and combined glucose and C-peptide criteria appeared to have less diagnostic value, with notably lower specificity. Among the ratio criteria, insulin-glucose ratio had the highest area under the ROC curve and exhibited a fair diagnostic performance, with both sensitivity and specificity over 90%. Therefore, insulin-glucose ratio can perhaps still be used as a clinical indicator in the differential diagnosis of insulinomas.
A recent German study rediscovered the diagnostic value of the amended insulin-glucose ratio for the biochemical diagnosis of insulinomas [5]. However, in our study, the diagnostic performances of the various amended ratios were not superior to those of simple ratios in terms of sensitivity and specificity. Although our study evaluated a limited number of patients, we found no good reason to use the amended insulin-glucose ratio instead of the simple insulin-glucose ratio. In addition, as we previously indicated, calculating amended insulin-glucose ratio is tricky when the plasma glucose level is less than or equal to 1.7 mmol/L.
Fasting for 3 days is a major inconvenience and hardship for patients undergoing evaluation of hypoglycemia. In 170 patients operated on for insulinoma who underwent prolonged fasts according to a standard protocol at the Mayo Clinic, it was reported that the fast was terminated within 12 hours in 33% of patients, 24 hours in 65%, 36 hours in 84%, 48 hours in 93%, and 72 hours in 99% [11]. Therefore, the ESCPG recommend a prolonged fast test of up to 72 hours to evoke a hypoglycemic episode [1]. However, all of the insulinoma patients ended the test within 48 hours in the abovementioned German study [5] and within 24 hours in our study. Although we cannot say that the duration of the prolonged fast test should be shortened based on the limited number of cases in the current study, the fact that the majority of patients with insulinoma terminated the test within 24 or 48 hours may help to guide patients who undergo evaluations for the causes of hypoglycemia.
Our study had several limitations. First, relatively few patients were included in the analysis. Given the low incidence of insulinoma, there are limited numbers of insulinoma patients, even in a tertiary hospital like our institution. A prospective cohort of insulinoma patients with a standardized protocol is needed for more comprehensive analysis. Second, the insulin and C-peptide assays were changed during the study period. However, because the detection limits of the insulin and C-peptide assays were similar and most of the patients were tested with specific insulin assays that are not reactive to human proinsulin, the potential effect of using different assays is probably minor.
In conclusion, among the various criteria for biochemical diagnosis of insulinoma, simultaneously fulfilling the glucose, insulin, and C-peptide criteria of the Endocrine Society exhibited the best diagnostic performance. Although its clinical usefulness for the diagnosis of insulinoma has long been questioned, the insulin-glucose ratio exhibited good sensitivity and specificity.
Figures and Tables
Fig. 2
Distribution of glucose, insulin, C-peptide, insulin-glucose ratio, C-peptide-glucose ratio, amended insulin-glucose ratio, and amended C-peptide-glucose ratio at discontinuation of the prolonged fast test. Scatter plots show the distributions of plasma glucose (A), insulin (B), C-peptide (C), insulin-glucose ratio (D), amended insulin-glucose ratio (E), C-peptide-glucose ratio (F), and amended C-peptide-glucose ratio (G). Dashed lines indicate the cutoff value from the Endocrine Society Clinical Practice Guidelines (A-C) or the cutoff value showing the highest Youden index in the receiver operating characteristic curve analysis of our current data (D-G). Closed circles, insulinoma; open circles, noninsulinoma.

Table 2
Comparison of Diagnostic Performance for Differentiating Insulinomas from Other Causes of Spontaneous Hypoglycemia

Units for insulin-glucose ratio and amended insulin-glucose ratio: (pmol/L)/(mmol/L). Units for C-peptide-glucose ratio and amended C-peptide-glucose ratio: (nmol/L)/(mmol/L). Youden index=sensitivity+specificity-1.
PPV, positive predictive value; NPV, negative predictive value; ESCPG, Endocrine Society Clinical Practice Guideline.
Table 3
Area under the ROC Curve for Each Ratio Criterion

Paired tests comparing any two ROC curves by the method described by DeLong et al. [10] showed no significant difference.
ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval.
References
1. Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ. Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009; 94:709–728.
2. Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005; 19:783–798.
3. McAuley G, Delaney H, Colville J, Lyburn I, Worsley D, Govender P, Torreggiani WC. Multimodality preoperative imaging of pancreatic insulinomas. Clin Radiol. 2005; 60:1039–1050.
4. Placzkowski KA, Vella A, Thompson GB, Grant CS, Reading CC, Charboneau JW, Andrews JC, Lloyd RV, Service FJ. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. J Clin Endocrinol Metab. 2009; 94:1069–1073.
5. Nauck MA, Meier JJ. Diagnostic accuracy of an "amended" insulin-glucose ratio for the biochemical diagnosis of insulinomas. Ann Intern Med. 2012; 157:767–775.
6. Vezzosi D, Bennet A, Fauvel J, Caron P. Insulin, C-peptide and proinsulin for the biochemical diagnosis of hypoglycaemia related to endogenous hyperinsulinism. Eur J Endocrinol. 2007; 157:75–83.
7. Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. Harrison's principles of internal medicine. 18th ed. New York: McGraw-Hill;2012.
8. Gardner DG, Shoback DM. Greenspan's basic and clinical endocrinology. 9th ed. New York: McGraw-Hill;2011.
9. Turner RC, Oakley NW, Nabarro JD. Control of basal insulin secretion, with special reference to the diagnosis of insulinomas. Br Med J. 1971; 2:132–135.
10. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845.
11. Service FJ, Natt N. The prolonged fast. J Clin Endocrinol Metab. 2000; 85:3973–3974.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download