Abstract
Background
In Iran, an iodine deficiency control program was initiated in 1989 by iodizing salt. Despite this program, goiters have remained an endemic condition in most parts of Iran. Thus, it is possible that other factors aside from iodine deficiency may contribute to endemic goiter. The aim of this study was to investigate the association between cobalt deficiency and endemic goiter in a region of Iran with a high prevalence of goiter.
Methods
A cross-sectional study was conducted among school children aged 9 to 11 years in the city of Kerman, Iran. In the first phase of the study, a multistage, proportional-to-size, cluster sampling method was used to screen 5,380 out of 29,787 students. After the screening phase, 170 students (130 goitrous and 40 nongoitrous) were randomly selected, and serum and urine specimens were obtained. We measured thyroid function, serum cobalt level, and urinary iodine excretion. Univariate and multiple logistic regression analyses were performed.
Results
The prevalence of grade 2 goiters was 34.8% (95% confidence interval [CI], 31.5 to 42.5), with both sexes being equally affected. The weight and body mass index of goitrous subjects was significantly lower (P<0.001) than those of nongoitrous subjects. The serum cobalt levels were lower in goitrous subjects than in nongoitrous subjects (4.4±2.9 µg/L vs. 6.4±2.7 µg/L). The urinary iodine levels were also lower in goitrous subjects than in nongoitrous subjects (198.3±108.3 µg/L vs. 270.2±91.1 µg/L). Multiple regression analysis showed that only cobalt deficiency, not iodine deficiency, significantly contributed to the presence of goiter (odds ratio, 0.78; 95% CI, 0.61 to 0.99; P=0.042).
Goiters are one of the most visible consequences of iodine deficiency [1], and Iran is a naturally iodine-deficient area. In 1969, the first epidemiologic survey on goiters in Iran revealed this high prevalence of iodine deficiency and goiters in most parts of Iran [2]. In 1989, an iodine deficiency control program, in which salt was iodized, was implemented in Iran. By 1996, as much as 93% of the rural and 97% of the urban population of the country were using iodized salt [3]. National reports in 2002 and 2008 declared that Iran's control program for iodine deficiency is ongoing [3,4], but nonetheless, goiters remain an endemic condition in most parts of Iran, especially among school-aged children [3,4,5,6,7]. Thus, it is feasible that factors other than iodine deficiency either directly or indirectly contribute to the etiology of endemic goiter [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. One possible contributing factor is cobalt, which is a rare trace element that has been shown to have goitrogenic effects [20,21,22]. Thus, the aim of this study was to investigate the goiter status in the city of Kerman, an area of endemic goiter in Iran, and to determine the association between serum cobalt levels and the presence of goiter.
This cross-sectional study was carried out among schoolchildren aged 9 to 11 years in the city of Kerman, the capital of Kerman, Iran. Sampling was performed from the records of all primary schools in the first and second districts of the city, which were obtained from Kerman's General Education Administration.
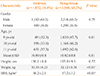
Notably, students with grade 2 goiters were assigned as cases and those with grade 0 goiters were assigned as controls. In this biphasic study, the first phase of study targeted 29,787 populations of 9 to 11-year-old students. Of these students, 5,380 were randomly selected using a multistage, proportional to population size sampling method. For all of the students, weight and height were measured, and body mass indexs (BMIs) were calculated. During the clinical thyroid examination, the thyroid size was recorded according to the World Health Organization/UNICEF/International Council for the Control of Iodine Deficiency Disorders criteria as grade "0" for no enlarged thyroid, grade "1" for a palpable and enlarged, but not visible thyroid, and grade "2" for an enlarged, visible goiter [23]. If the grading of a thyroid fell between grades, the immediate lower grade was recorded. In order to monitor the accuracy of the examinations, the thyroid size of 10% of the samples, which were randomly selected, were re-examined by the first author (MS). In addition, continual training sessions were performed for the examiners before and during the study in order to ensure proper examination.
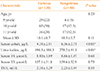
In the second phase of the study, a subgroup of students was randomly selected among participating students from the first phase. Serum samples from 130 randomly selected goitrous students and 40 randomly selected nongoitrous students were then collected to evaluate thyroid function tests, urinary iodine excretion, and serum cobalt levels. Thyroid stimulating hormone (TSH), thyroxine (T4), and triiodothyronine (T3) were measured with a radioimmunoassay technique using an IRMA kit (Kavoshyar, Tehran, Iran). Urinary iodine excretion was measured in causal urine samples by the digestion method. Serum cobalt was determined using the electrothermal atomic absorption spectrometric method described by Todorovska et al. [24]. Serum cobalt levels of 1.2 to 36 µg/L, TSH levels of 0.5 to 6 mU/L, and serum T4 levels of 4.6 to 11 nmol/L were considered to be normal. Iodine deficiency was defined as having a urinary iodine excretion less than 100 µg/L.
All subjects and their parents or guardians were briefed on the study objectives during a school visit, and parents or guardians gave their written informed consent. The ethics committee of Kerman's University of Medical Sciences approved this study protocol.
All continuous data are presented as the mean±SD and were compared using independent t tests. Categorical data are shown as numbers and percentages and were compared using the chi-square test. Multivariate logistic regression was used to calculate the crude and adjusted odds ratios (ORs) for the association between goiter (as the dependent variable) and other independent variables including age, sex, T3, T4, urine iodine level, and cobalt level. The crude and adjusted ORs with 95% confidence intervals (CI) are presented. Comparisons with P values less than 0.05 were considered statistically significant. All analyses were conducted using Stata/SE version 11 statistical software (StataCorp, College Station, TX, USA).
Of the 5,380 recruited students, 3,401 were males, and 1,979 were females. The prevalence of grade 2 goiters was 34.8% (95% CI, 31.5 to 42.5), and both sexes were affected equally (34.8% for each sex). There were no significant differences in height between the two groups, but weight and BMI were significantly lower in the goitrous patients (P<0.001) compared to the nongoitrous patients. A summary of the study population demographics are shown in Table 1.
The characteristics of the subgroup population are presented in Table 2. In the subgroup population, serum cobalt levels were significantly lower in goitrous subjects than in nongoitrous subject (4.4±2.9 µg/L vs. 6.4±2.7 µg/L, P<0.001). The excretion of urinary iodine were also significantly lower in goitrous subjects than in nongoitrous subjects (198.3±108.3 µg/L vs. 270.2±91.1 µg/L, P<0.001).
As seen in Table 3, the initial univariate analysis showed cobalt and iodine deficiency to be associated with a statistically significant increase in the risk of endemic goiter, with an OR of 1.23 (95% CI, 1.10 to 1.41; P=0.002) and an OR of 1.01 (95% CI, 1.00 to 1.01; P=0.047), respectively. After adjustments, only cobalt deficiency was significantly associated with the presence of goiter, with an OR of 1.28 (95% CI, 1.02 to 1.64; P=0.04). Notably, iodine deficiency was not a significant contributor to endemic goiter (P=0.11).
In this cross-sectional study in the city of Kerman, we demonstrated the rate of grade 2 goiter to be 34.8% for both genders. The province of Kerman is located in Central Iran, an area previously known to be a hyperendemic region of iodine deficiency and goiter. Seven years after the commencement of the national iodine deficiency control program, the prevalence of goiter among schoolchildren in the province of Kerman was still as much as 59% [5]. Although, in 2001, the goiter prevalence decreased to 31.6% in this population [6]. The differences between our results and the results of previous studies are likely due to differences in the target populations. In the mentioned studies, the target population was 8- to 10-year-old in the province of Kerman, but we enrolled 9- to 11-year-old children in the city of Kerman.
Cobalt is a trace element with properties similar to iron and nickel. This trace element is a well-known component of vitamin B12, essential for B12's biosynthesis, which is necessary for producing red blood cells and maintaining the nervous system. However, excessive cobalt intake causes goiter and reduces thyroid activity [25]. Since our region is a hyperendemic goiter area, this study was designed to compare the prevalence of cobalt excess as a cause of goiter between goitrous and non-goitrous children and to assess the relationship between serum cobalt levels and thyroid hormones, as well.
However, our results demonstrated a significant positive association between cobalt deficiency and the presence of endemic goiter. On the other hand, our analyses showed no significant association between goiter and iodine deficiency. The iodine deficiency control program of Iran, which consisted of salt iodization programs, has been in place since 1989. Moreover, previous studies have shown adequate iodine intake in school-age children in Kerman since 1996 [5,6]. Despite years of adequate iodine intake in Kerman, the prevalence of goiter has not decreased appreciably, and many regional studies investigating the effects of different factors, mainly immune and dietary, as the cause of endemic goiter confirm this finding [8,9,10,11,12,13,14,15,16,17,18,19].
Currently, the literature regarding cobalt and thyroid function, especially the presence of goiter, is scarce. Despite the scarcity of specific literature, the consensus of the literature suggests that increased levels of cobalt may confer damaging and goitrogenic effects. The first reports of the goitrogenic action of cobalt came out in 1955 in animal experiments and in human subjects [20]. Additionally, research shows that the lung, skin, thyroid, heart, and bone marrow are potential target organs of cobalt [21]. In 1961, three cases of cobalt-induced goiter were reported in the pediatric ward of Vanderbilt University Hospital [20]. One proposed mechanism by which cobalt may induce goitrogenic effects is via inhibition of one or more of the enzymatic reactions at different levels of thyroid hormone formation [20]. Occupational exposure to cobalt was evaluated in female plate painters in Denmark and in cobalt refinery workers [21]. In the Danish study, there was no correlation between urinary cobalt and serum TSH or thyroid volume [20]. In the refinery worker study, a slight interference with thyroid metabolism (decreased T3, T4, and increased TSH) was found in the exposed group. However, no cases of hypothyroidism were found [21].
As our results show, it is conceivable that iodine deficiency may no longer be the primary cause of goiter in Kerman. Cobalt deficiency may be one of the reasons for the persistence of goiter in this region, despite the successful iodine supplementation programs. Our results showed a greater deficit in cobalt levels in the goitrous group compared to the nongoitrous group, which is in contrast to the available literature that suggests that increased levels of cobalt tend to cause goitrogenic effects. However, the underlying mechanism and pathogenesis of the effects of cobalt, be it in excess or deficiency, on the thyroid is not yet known, suggesting a potential role for additional contributing factors, and suggesting a need for further, larger-scale investigation.
Figures and Tables
ACKNOWLEDGEMENTS
This study was a part of a common project and sponsored by the Kerman University of Medical Sciences and Kerman High Technology and Environmental Sciences. The authors thank all participant of this study. We also thank medical students who examined the students. The authors thanks Dr Kholamhosseinian, Dr Moinee, and Mr Shokouhi for assistance in the project. The author would like to thank the Farzan Institute for Research and Technology for its technical assistance.
References
1. Dunn JT. Seven deadly sins in confronting endemic iodine deficiency, and how to avoid them. J Clin Endocrinol Metab. 1996; 81:1332–1335.
2. Emami A, Shahbazi H, Sabzevari M, Gawam Z, Sarkissian N, Hamedi P, Hedayat H. Goiter in Iran. Am J Clin Nutr. 1969; 22:1584–1588.
3. Azizi F, Sheikholeslam R, Hedayati M, Mirmiran P, Malekafzali H, Kimiagar M, Pajouhi M. Sustainable control of iodinedeficiency in Iran: beneficial results of the implementation of the mandatory law on salt iodization. J Endocrinol Invest. 2002; 25:409–413.
4. Azizi F, Mehran L, Sheikholeslam R, Ordookhani A, Naghavi M, Hedayati M, Padyab M, Mirmiran P. Sustainability of a well-monitored salt iodization program in Iran: marked reduction in goiter prevalence and eventual normalization of urinary iodine concentrations without alteration in iodine content of salt. J Endocrinol Invest. 2008; 31:422–431.
5. Delshad H, Sheikholeslam R, Mirmiran P, Abdolhossaini G, Hedayati M, Azizi F. Goiter survey and urinary Iodine concentration in school children aged 8 to 10 year of Kerman province in 1996. J Kerman Univ Med Sci. 2002; 9:1–6.
6. Sheikholeslam R, Aflatonian MR, Toori K, Abdollahi Z, Samadpoor K, Azizi F. Prevalence of Goiter and urinary Iodine content in schoolchildren of Kerman (Iran) in 2001. J Kerman Univ Med Sci. 2006; 13:15–21.
7. Rezvanian H, Aminorroaya A, Majlesi M, Amini A, Hekmatnia A, Kachoie A, Amini M, Emami J. Thyroid size and iodine intake in iodine-repleted pregnant women in Isfahan, Iran. Endocr Pract. 2002; 8:23–28.
8. Rezvanfar MR, Farahany H, Chehreiy A, Nemati M, Rostamy S, Karimy E. Urinary iodine excretion and antiperoxidase enzyme antibody in goitrous and healthy primary school children of Arak, Iran. J Endocrinol Invest. 2007; 30:274–278.
9. Bazrafshan HR, Mohammadian S, Ordookhani A, Farhidmehr F, Hedayati M, Abdolahi N, Azizi F, Braverman LE, Pearce EN. Prevalence of goiter among schoolchildren from Gorgan, Iran, a decade after national iodine supplementation: association with age, gender, and thyroperoxidase antibodies. J Endocrinol Invest. 2005; 28:727–733.
10. Brauer VF, Below H, Kramer A, Führer D, Paschke R. The role of thiocyanate in the etiology of goiter in an industrial metropolitan area. Eur J Endocrinol. 2006; 154:229–235.
11. Knudsen N, Bulow I, Laurberg P, Ovesen L, Perrild H, Jorgensen T. Association of tobacco smoking with goiter in a low-iodine-intake area. Arch Intern Med. 2002; 162:439–443.
12. Pontikides N, Krassas GE. Influence of cigarette smoking on thyroid function, goiter formation and autoimmune thyroid disorders. Hormones (Athens). 2002; 1:91–98.
13. Brauer VF, Schweizer U, Kohrle J, Paschke R. Selenium and goiter prevalence in borderline iodine sufficiency. Eur J Endocrinol. 2006; 155:807–812.
14. Hashemipour M, Amini M, Aminorroaya A, Dastjerdi MI, Rezvanian H, Kachoei A, Moaddab MH, Mohammadi M, Kelishadi R, Amini Z, Haghighi S, Shojaee-Moradie F. High prevalence of goiter in an iodine replete area: do thyroid auto-antibodies play a role? Asia Pac J Clin Nutr. 2007; 16:403–410.
15. Dabbaghmanesh MH, Sadegholvaad A, Ejtehadi F, Ranjbar-Omrani G. The role of iron deficiency in persistent goiter. Arch Iran Med. 2008; 11:157–161.
16. Siavash Dastjerdi M, Hashemipour M, Rezvanian H, Kazemi F, Najafian A, Mohammady M, Aminorroaya A, Amini M, Kachuei A, Hassan Moaddab M. Iron deficiency in goitrous schoolchildren of Semirom, Iran. Horm Res. 2006; 66:45–50.
17. Eftekhari MH, Simondon KB, Jalali M, Keshavarz SA, Elguero E, Eshraghian MR, Saadat N. Effects of administration of iron, iodine and simultaneous iron-plus-iodine on the thyroid hormone profile in iron-deficient adolescent Iranian girls. Eur J Clin Nutr. 2006; 60:545–552.
18. Kimiagar M, Azizi F, Navai L, Yassai M, Nafarabadi T. Survey of iodine deficiency in a rural area near Tehran: association of food intake and endemic goitre. Eur J Clin Nutr. 1990; 44:17–22.
19. Zimmermann MB, Jooste PL, Mabapa NS, Schoeman S, Biebinger R, Mushaphi LF, Mbhenyane X. Vitamin A supplementation in iodine-deficient African children decreases thyrotropin stimulation of the thyroid and reduces the goiter rate. Am J Clin Nutr. 2007; 86:1040–1044.
20. Swennen B, Buchet JP, Stanescu D, Lison D, Lauwerys R. Epidemiological survey of workers exposed to cobalt oxides, cobalt salts, and cobalt metal. Br J Ind Med. 1993; 50:835–842.
21. Chamberlain JL 3D. Thyroid enlargement probably induced by cobalt: a report of 3 cases. J Pediatr. 1961; 59:81–86.
22. Prescott E, Netterstrom B, Faber J, Hegedüs L, Suadicani P, Christensen JM. Effect of occupational exposure to cobalt blue dyes on the thyroid volume and function of female plate painters. Scand J Work Environ Health. 1992; 18:101–104.
23. Delange F. The disorders induced by iodine deficiency. Thyroid. 1994; 4:107–128.
24. Todorovska N, Karadjova I, Arpadjan S, Stafilov T. Electrothermal atomic absorption spectrometric-determination of cobalt in human serum and urine. Acta Pharm. 2003; 53:83–90.
25. Strachan S. Trace element. Curr Anaesth Crit Care. 2010; 21:44–48.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download