Abstract
Background
Fine-needle aspiration (FNA) of the thyroid is a widely accepted confirmatory test for thyroid cancer with high sensitivity and specificity. FNA is a simple procedure that is learned by many clinicians to enable accurate diagnosis of thyroid cancer. However, it is assumed that because the FNA test is a relatively simple procedure, its cytologic results are reliable regardless of the operator's experience. The aim of this study was to evaluate the differences in the diagnostic indices of FNA between operators with different levels of experience.
Methods
A total of 694 thyroid FNA specimens from 469 patients were reviewed, and were separated based on the experience of the clinicians who performed the procedure. One hundred and ninety were categorized in the experienced group, and 504 in the inexperienced group. All FNA results were then compared with histological data from surgically resected specimens, and the sample adequacy and diagnostic accuracy of the groups were compared.
Results
The age, gender, and nodule size and characteristics were similar in both groups. The sample adequacy rate was not significantly different between the experienced and nonexperienced groups (96.3% vs. 95.4%, P=0.682). However, the non-experienced group had a higher false-negative rate than the experienced group (6.4% vs. 17.2%, P=0.038), and the sensitivity of the FNA test also tended to be lower in the nonexperienced group (95.6% vs. 88.9%, P=0.065).
Conclusion
These results suggest that FNA operators who have less experience may miss cases of thyroid cancer by performing the procedure incorrectly. As such, the experience of the FNA operator should be considered when diagnosing thyroid cancer. When clinicians are being trained in FNA, more effort should be made to increase the accuracy of the procedure; therefore, enhanced teaching programs and/or a more detailed feedback system are recommended.
Thyroid nodules are detected at a rate of 30% to 50% by ultrasonographic examination in adults [1,2,3,4,5]. Fine-needle aspiration (FNA) is commonly used as the first-line screening test for patients with thyroid nodules, and is the most reliable diagnostic test because of its high sensitivity and specificity [6,7,8]. The routine use of FNA to evaluate thyroid nodules or neck masses has resulted in a decrease in unnecessary surgeries, and an increase in the detection rate of malignant thyroid lesions [9,10]. FNA is a simple and safe technique, and, as such, many clinicians seek training in the procedure to allow them to evaluate thyroid nodules. However, some factors make obtaining adequate specimens difficult, for example, small nodule size, difficult nodule location, calcification or cystic changes of the nodule, and hypervascularity [11,12,13]. Sufficient operator experience may therefore affect the incidence of false negative results. However, the effect of operator experience on successful diagnosis of thyroid cancer has not been evaluated. A recent study reported no differences in sample adequacy, pain-scale ratings, or complications between two operators with different levels of experience; the experienced operator had performed more than 12,000 FNA procedures over 10 years, compared to a less experienced operator with approximately 500 procedures [14]. One conclusion from this study could therefore be that the experience of the operator has little effect on the cytological results or adequacy rate. However, sample adequacy does not reflect the accuracy of needle localization, which is one of the most important differences between experienced and inexperienced operators. As such, falsely targeted specimens may be misinterpreted as being adequately sampled. For appropriate comparison of FNA accuracy, it is necessary to directly compare the cytological results from FNA with the histopathological results from surgically resected specimens. The aim of this study was therefore to investigate whether there were differences in the diagnostic performance of FNA between operators with different levels of experience.
We retrospectively reviewed the medical records of patients who underwent total or hemithyroidectomy at the Kyung Hee University Medical Center between January 2011 and February 2013. A total of 728 patients were identified. Among these, patients who underwent FNA in the Department of Endocrinology were included in the study. Two hundred and fifty-nine patients who underwent FNA at another hospital or department were excluded because of the uncertainty of performer's experience. A total of 694 nodules from 469 patients were analyzed.
Aspiration was performed by clinicians in the Department of Endocrinology using a 25-gauge needle with no syringe. A high-resolution ultrasonography (US) modality was used for guidance during FNA (iU 22, Philips Medical Systems, Bothell, WA, USA). After nodule localization using US, the needle was introduced perpendicular to the transducer. When the needle reached the target zone, the operator gently moved the needle up and down, and the needle was withdrawn when an appropriate amount of material had filled the needle hub. All of these processes were performed two to four times per nodule. Specimens were smeared, fixed in 95% ethanol, and examined after Papanicolaou staining.
The results of cytologic evaluation were classified according to the Bethesda System for Reporting Cytopathology, as follows [15]: nondiagnostic (fewer than six clusters of thyroid follicular cells), benign (including benign follicular nodule, lymphocytic thyroiditis, and granulomatous thyroiditis), atypia of undetermined significance (AUS) or follicular lesion of undetermined significance, follicular neoplasm, suspicious for malignancy, or malignant. Pathological specimens from thyroidectomy were stained with hematoxylin and eosin, and histologic diagnoses were performed based on standard procedures. If there was any discrepancy between the cytological and histological results, the specimen was reevaluated, and the cytological and histological results were compared.
Kyung Hee University Medical Center is a teaching hospital, and includes a training system for thyroid FNA. Skilled operators teach and supervise trainees during their training session. Based on this system, all aspirated nodules were divided into two groups according to the experience of the FNA operator. Diagnostic indices (sensitivity, specificity, positive predictive value [PPV], negative predictive value [NPV], and accuracy) of each group were then compared. The experienced group consisted of five operators with a minimum experience level of 1,000 FNA cases; in this study, they examined 190 nodules from 130 patients. In the inexperienced group, which consisted of 13 operators with experience involving less than 300 FNA cases, 504 nodules from 339 patients were examined.
To calculate FNA diagnostic indices, all cytological and histological data were classified into positive and negative groups. The results of FNA were considered positive when they were either suspicious or definitely positive for malignancy, whereas nondiagnostic or benign results were considered negative. AUS and follicular neoplasms were excluded from analysis, because these cytologic results show a variable malignancy rate, meaning that they could not be expressed as cytologically 'positive' or 'negative.' The histological results were considered positive when they were malignant, and all other histological results were classified as negative. The diagnostic performances of the experienced and inexperienced groups were calculated separately, and the results were compared.
Data were analyzed using SPSS version 18.0 (IBM Co., Armonk, NY, USA), and all continuous variables were reported as mean±SD. Clinical characteristics were compared using the Student t test, and the differences in diagnostic rates were assessed using the chi-square or Fisher exact test. Variables that were not normally distributed were analyzed using non-parametric tests. Nodule characteristics were compared by one-way analysis of variance. A value of P<0.05 was considered to indicate statistical significance.
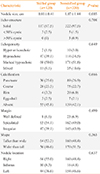
The clinical performance of the experienced and inexperienced groups was assessed (Table 1). The age and gender ratios of the groups were not significantly different. The mean nodule size was comparable, 1.26±1.10 cm in the experienced group and 1.43±1.57 cm in the inexperienced group. Next, the characteristics of nodules histologically confirmed as malignant in each group were compared (Table 2). The nodule size was smaller in the experienced group compared with the inexperienced group (0.81±0.41 cm vs. 1.07±1.44 cm, P=0.003). However, no significant differences were observed in any other measured parameters, including echo structure, echogenicity, calcification, margin, shape, or location. The cytological and histological results of all 694 nodules are listed in Supplemental Table S1.
The experienced group examined 190 nodules, and the inexperienced group assessed 504 nodules. Nondiagnostic results were obtained in seven cases from the experienced, and 23 from the inexperienced group. The sample adequacy rate was therefore 96.3% in the experienced group and 95.4% in the inexperienced group, and no significant differences were observed (P=0.682) (Table 3).
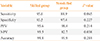
The cytological and histological correlations of the experienced and inexperienced groups are shown in Supplemental Tables S2, S3. Based on these data, the diagnostic indices of both groups were calculated and compared (Table 4). The sensitivities were 95.6% and 88.9% in the experienced and inexperienced groups, respectively (P=0.065). The diagnostic specificities were 93.5% and 97.4% in the experienced and inexperienced groups, respectively (P=0.227). PPV was 95.6% in the experienced and 98.4% in the inexperienced group (P=0.214), while NPV was significantly higher in the experienced group (93.5% vs. 82.7%, P=0.038). The inexperienced group therefore had a higher false negative diagnostic rate than the experienced group (17.3% vs. 6.5%). Finally, the accuracy rate was 94.8% in the experienced and 91.9% in the inexperienced group (P=0.284).
Malignant nodules were classified into two groups: false-negative nodules in which the cytological results were benign or non-diagnostic but the histological results were malignant, and true-positive nodules in which the cytological and histological results were both malignant. Overall, there were 36 false-negative and 348 true-positive nodules. The nodule size and characteristics of the two groups were then compared (Table 5). The mean nodule sizes were 0.92±0.83 and 1.01±1.31 cm in the false-negative and true-positive groups, respectively (P=0.690). The nodule characteristics of both groups were similar, with the exception of echo structure: in the false-negative group, there was a higher percentage of predominantly (more than 50%) cystic nodules (5.5% vs. 0.2%, P=0.019).
The prevalence of thyroid cancer is increasing in many parts of the world, although this is likely due to increased detection of small tumors based on enhanced diagnostic procedures [16,17,18,19,20]. FNA is safe, easy to perform, and the most important test for diagnosis of thyroid cancer; as a result, increasing numbers of clinicians are learning the FNA procedure. The purpose of the present study was to determine the effect of operator experience on FNA performance. There was no difference in the sample adequacy rate between experienced and inexperienced operators. However, more false-negative results occurred when less-experienced operators performed FNA. No differences in nodule characteristics were observed between groups, except with regard to nodule size. However, the difference in nodule size between groups was significant only when malignant nodules were analyzed, and not when all nodules were assessed. This may be because an experienced operator examined smaller nodules when malignancy was suspected.
In addition, we reviewed the characteristics of false-negative nodules to identify factors that affected the cytological results. We found a difference in echo structure, specifically that the percentage of predominantly cystic nodules was increased in false-negative compared to true-positive nodules. However, because of the small number of cases (two in the false-negative group, one in the true-positive group), it was not possible to determine whether this was of clinical significance. Whether cystic changes in the nodule relate to cytological findings could therefore not be determined in this study. We did not find any significant differences between the groups in other nodule characteristics, including size, echogenicity, calcification, margin shape, and nodule location.
In a previous study, there was no significant difference in the cytological adequacy of two radiologists with different levels of experience [14]. This is consistent with our findings, although our data suggest that less-experienced operators obtain more false negative results despite acquiring an adequate amount of specimen. These observations may be related to poor needle localization. When the operator performs FNA, they must be able to see the needle tip in the nodule. Although this is not difficult, less-experienced operators can have trouble accurately localizing the needle tip, especially when a nodule is calcified, cystic, small, or in a difficult location (Figs. 1, 2). Because of this, many articles have recommended that experienced FNA operators perform the procedure [10,21,22,23]; however, to our knowledge, few studies have compared the diagnostic accuracy of FNA carried out by experienced and inexperienced operators. The aim of the present study was to assess this difference. Because FNA is such a simple procedure, many clinicians, even the operators themselves, often overlook the fact that experience may affect the cytological results. False-negative FNA results of malignant nodules can lead to repeat examination or misdiagnosis of thyroid cancer. The findings of this study therefore have significant implications.
To decrease false negative procedures, clinicians who are new to the procedure should make every effort to verify the position of the needle tip during each FNA exam, particularly for difficult nodules. Practicing on thyroid models or performing the procedure under the supervision of an experienced operator with timely feedback is therefore recommended. FNA instruction during residency training or fellowship programs may be a good method by which this can be achieved [24].
The limitations of this study should be considered. First, the number of nodules was limited, so it was difficult to draw significant conclusions when comparing the characteristics of false negative and true positive nodules. Second, the nodule location was classified as right, left, or isthmus; 'difficult' positions, such as deep nodules, those next to large vessels, or those in the uppermost/lowermost position of the thyroid lobe were not considered. Third, the diagnostic indices affected by prevalence rate, PPV and NPV may be biased because of the high cancer prevalence rate of this study. False negative cytology is only important when nodules are sonographically indeterminate or suspicious for malignancy. The malignancy prevalence rates of sonographically suspicious nodules in previous studies were similar to that of this study, so the PPV and NPV of this study may have clinical significance [25,26,27]. Finally, diagnostic indices varied among operators, especially in the inexperienced group. It is therefore difficult to generalize the results of this study to all clinicians who are beginning to learn the procedure; however, the inexperienced group contained 13 operators, so the results may have clinical significance.
In conclusion, thyroid FNA operators who have carried out less than 300 FNA procedures obtained a greater number of false-negative results. Clinicians learning the FNA procedure should attempt to decrease the incidence of false-negative results and consequent misdiagnosis of thyroid cancer by enrolling in teaching programs and/or seeking out supervision and feedback from expert operators.
Figures and Tables
Fig. 1
Examples of difficult nodules (arrows). (A) Deep, small nodule, (B) nodule in the uppermost part of the right thyroid lobe, (C) dense, calcified nodule.

Fig. 2
An example of a potentially erroneous needle localization. (A) A bright echo (arrow) is visible in the ill-defined hypoechoic nodule of the right thyroid lobe. (B) However, the needle tip was localized in the deep portion of the lobe (arrow). The bright echo seen in (A) is in fact the needle shaft.

References
1. Kang HW, No JH, Chung JH, Min YK, Lee MS, Lee MK, Yang JH, Kim KW. Prevalence, clinical and ultrasonographic characteristics of thyroid incidentalomas. Thyroid. 2004; 14:29–33.
2. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB, Goellner JR, Hay ID, Hertzberg BS, Intenzo CM, Jeffrey RB, Langer JE, Larsen PR, Mandel SJ, Middleton WD, Reading CC, Sherman SI, Tessler FN. Society of Radiologists in Ultrasound. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005; 237:794–800.
3. Lim DJ, Kim JY, Baek KH, Kim MK, Park WC, Lee JM, Kang MI, Cha BY. Natural course of cytologically benign thyroid nodules: observation of ultrasonographic changes. Endocrinol Metab (Seoul). 2013; 28:110–118.
4. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
5. Gul K, Ersoy R, Dirikoc A, Korukluoglu B, Ersoy PE, Aydin R, Ugras SN, Belenli OK, Cakir B. Ultrasonographic evaluation of thyroid nodules: comparison of ultrasonographic, cytological, and histopathological findings. Endocrine. 2009; 36:464–472.
6. Baloch ZW, Sack MJ, Yu GH, Livolsi VA, Gupta PK. Fine-needle aspiration of thyroid: an institutional experience. Thyroid. 1998; 8:565–569.
7. Cap J, Ryska A, Rehorkova P, Hovorkova E, Kerekes Z, Pohnetalova D. Sensitivity and specificity of the fine needle aspiration biopsy of the thyroid: clinical point of view. Clin Endocrinol (Oxf). 1999; 51:509–515.
8. Castro MR, Gharib H. Thyroid fine-needle aspiration biopsy: progress, practice, and pitfalls. Endocr Pract. 2003; 9:128–136.
9. Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993; 118:282–289.
10. Mittendorf EA, Tamarkin SW, McHenry CR. The results of ultrasound-guided fine-needle aspiration biopsy for evaluation of nodular thyroid disease. Surgery. 2002; 132:648–653.
11. Kim MJ, Kim EK, Park SI, Kim BM, Kwak JY, Kim SJ, Youk JH, Park SH. US-guided fine-needle aspiration of thyroid nodules: indications, techniques, results. Radiographics. 2008; 28:1869–1886.
12. Belfiore A, La Rosa GL. Fine-needle aspiration biopsy of the thyroid. Endocrinol Metab Clin North Am. 2001; 30:361–400.
13. Kim DW. How to do it: ultrasound-guided fine-needle aspiration of thyroid nodules that commonly result in inappropriate cytology. Clin Imaging. 2013; 37:1–7.
14. Lee YJ, Kim DW, Jung SJ. Comparison of sample adequacy, pain-scale ratings, and complications associated with ultrasound-guided fine-needle aspiration of thyroid nodules between two radiologists with different levels of experience. Endocrine. 2013; 44:696–701.
15. Mufti ST, Molah R. The bethesda system for reporting thyroid cytopathology: a five-year retrospective review of one center experience. Int J Health Sci (Qassim). 2012; 6:159–173.
16. Agate L, Lorusso L, Elisei R. New and old knowledge on differentiated thyroid cancer epidemiology and risk factors. J Endocrinol Invest. 2012; 35:6 Suppl. 3–9.
17. Malandrino P, Pellegriti G, Attard M, Violi MA, Giordano C, Sciacca L, Regalbuto C, Squatrito S, Vigneri R. Papillary thyroid microcarcinomas: a comparative study of the characteristics and risk factors at presentation in two cancer registries. J Clin Endocrinol Metab. 2013; 98:1427–1434.
18. Malandrino P, Scollo C, Marturano I, Russo M, Tavarelli M, Attard M, Richiusa P, Violi MA, Dardanoni G, Vigneri R, Pellegriti G. Descriptive epidemiology of human thyroid cancer: experience from a regional registry and the "volcanic factor". Front Endocrinol (Lausanne). 2013; 4:65.
19. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.
20. Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992-2006. Thyroid. 2011; 21:125–134.
21. Cramer H. Fine-needle aspiration cytology of the thyroid: an appraisal. Cancer. 2000; 90:325–329.
22. Ravetto C, Colombo L, Dottorini ME. Usefulness of fine-needle aspiration in the diagnosis of thyroid carcinoma: a retrospective study in 37,895 patients. Cancer. 2000; 90:357–363.
23. Giard RW, Hermans J. Use and accuracy of fine-needle aspiration cytology in histologically proven thyroid carcinoma: an audit using a national nathology database. Cancer. 2000; 90:330–334.
24. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol. 1997; 17:239–247.
25. Hambly NM, Gonen M, Gerst SR, Li D, Jia X, Mironov S, Sarasohn D, Fleming SE, Hann LE. Implementation of evidence-based guidelines for thyroid nodule biopsy: a model for establishment of practice standards. AJR Am J Roentgenol. 2011; 196:655–660.
26. Park JY, Lee HJ, Jang HW, Kim HK, Yi JH, Lee W, Kim SH. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid. 2009; 19:1257–1264.
27. Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002; 178:687–691.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download