Abstract
Background
The tubby protein has a motif that might be relevant for its action in the insulin signaling pathway. Previous studies have indicated that tubby undergoes phosphorylation on tyrosine residues in response to several stimuli and is known to localize in the nucleus as well as in the plasma membrane. However, the relationship between phosphorylation and nuclear translocation is not well understood. Here, we report that insulin directly phosphorylates tubby, which translocates into the nucleus.
Methods
The effects of insulin on Tubby were performed with Western blot. The immunoprecipitation and confocal microscopy were performed to prove phosphorylation and nuclear translocation.
Results
Mutation study reveals that tyrosine residue 464 of tubby gene (Tub) is a phosphorylation site activated by insulin. In addition, major portions of tubby protein in the plasma membrane are translocated into the nucleus after insulin treatment. Tyrosine kinase inhibitor pretreatment blocked insulin-induced tubby translocation, suggesting that phosphorylation is important for nuclear translocation. Moreover, mutant tyrosine residue 464 did not translocate into the nucleus in respond to insulin. These findings demonstrate that insulin phosphorylates tyrosine residue 464 of Tub, and this event is important for insulin-induced tubby nuclear translocation.
The tubby strain of obese mice provides one of the few defined models for adult-onset obesity [1]. The tubby gene (Tub), which is highly expressed in the paraventricular nucleus of the hypothalamus and several other brain regions, was identified by isolating the genetic locus that transmits this autosomal recessive obesity syndrome [2,3]. Tubby mice have a naturally occurring splice site mutation at the junction of the 3' codon region. Targeted deletion of the Tub results in a phenotype identical to that of the naturally occurring mutant (mt) [4], indicating that the tubby obesity syndrome indeed arises from a loss of function.
The tubby protein is a member of a homologous family with four members (tubby and TULP s 1 to 3) encoded in the human genome and with others present in various multicellular organisms [5,6]. The striking feature of the Tub family of proteins is a highly conserved C-terminus. Specifically, the last 250 amino acids are 55% to 95% identical across species [7], suggesting that this domain may perform a key function. In contrast, the N-terminal portion of tubby and tubby-like proteins is poorly conserved. These data indicate that tubby may have a role in insulin signaling, possibly functioning as a mediator of insulin. These findings may have implications for studies investigating a tubby signal and the role of tubby in the insulin signaling pathway. Tub encodes a highly hydrophilic protein containing putative tyrosine phosphorylation and Src homology 2 (SH2)-docking sites [8]. Phosphotyrosine-mediated interactions with SH2-containing signaling proteins have been shown to play an important role in intracellular signal transduction [9]. To date, however, many questions remain about the role of tubby and its signal pathway.
Insulin's action in the central nervous system (CNS) has been implicated in the regulation of energy intake and expenditure. Administration of insulin directly into the CNS leads to a reduction in food intake and body weight in rats. The insulin receptor, a member of a large family of transmembrane receptor protein tyrosine kinases, is widely distributed in the CNS, and its hypothalamic expression matches that of the leptin receptor and Tub [10]. Binding of insulin to its cell surface receptor initiates a cascade of events that leads to the phosphorylation of downstream targets, including insulin receptor substrate-1 (IRS-1). IRS-1 is an adaptor protein that links upstream kinases to downstream signaling pathways. Tyrosine phosphorylation of IRS-1 bound to various SH2-containing signaling molecules links the insulin receptor to a myriad of signaling pathways previously implicated in the mediation of cellular responses to insulin signals. By analogy to IRS-1, we postulated that tubby might function as a downstream signal of the insulin receptor.
The tubby domain includes a motif suggestive of its action in insulin signaling. As discussed earlier, Tub encodes a highly hydrophilic protein containing putative tyrosine phosphorylation and SH2-docking sites. Recently, it was reported that Tub was phosphorylated by insulin receptors, Abl, and JAK2 but not by epidermal growth factor receptor (EGFR) or Src. However, little is known about the phosphorylation site in tubby or the role of phosphorylation in cell signaling.
Here, we report that tubby was phosphorylated on a specific tyrosine residue that is important for nuclear translocation of the tubby protein by insulin. Although the specific mechanisms involved are not well understood, this study suggests a possible role of the tubby protein in the insulin signaling pathway. Our findings of insulin-induced phosphorylation and insulin-mediated nuclear translocation provide support for this hypothesis. In the future, further concentrated study is necessary to explain the underlying molecular mechanisms of tubby activation in insulin-related pathophysiological phenomena such as obesity and type 2 diabetes mellitus.
HIRcB, a rat fibroblast cell line that overexpresses the human insulin receptor, was cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum in a humid atmosphere of 5% CO2/95% O2 at 37℃. COS-7 cells were maintained in DMEM supplemented with 10% heat-inactivated bovine calf serum. Cells were planted on a 6- or 10 cm tissue culture dish that was precoated with poly-L-lysine (10 g/mL) and were grown for 1 to 2 days until they reached 50% to 80% confluency. Cells then were placed in serum-free DMEM for 12 to 18 hours before treatment with 10 ng/mL of EGFR.
A bright green fluorescent protein (GFP) was attached to the carboxyl-terminus of tubby by standard recombinant techniques using the pEGFP-C1 vector (Invitrogen, Carlsbad, CA, USA).
The cells were grown in 6-well plates and, once at 60% to 70% confluency, were serum-starved for 24 hours before treatment at 37℃ with selected agents. The media were then aspirated, and the cells were washed twice with ice-cold phosphape buffer saline (PBS) and lysed in 100 L of lysis buffer. The samples were briefly sonicated, heated at 95℃ for 5 minutes, and centrifuged for 5 minutes. The supernatant was electrophoresed on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 8% to 16%) gradient gels and transferred to a polyvinylidene difluoride membrane. The blots were incubated overnight at 4℃ with primary antibodies and washed six times with Tris-buffered saline with 0.1% tween 20 before being probed with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. The blots were then visualized with ECL (Amersham Biosciences, Piscataway, NJ, USA). In some cases, the blots were stripped and reprobed with other antibodies.
After treatment with inhibitors and drugs, the cells were placed on ice, washed twice with ice-cold PBS, lysed in lysis buffer containing 50 mM Tris-HCl at pH 8.0, 100 mM NaCl, 20 mM NaF, 10 mM sodium pyrophosphate, 5 mM ethylenediaminetetraacetic acid, 1% Nonidet P-40, 10 g/mL aprotinin, 10 g/mL leupeptin, 10 g/mL soybean trypsin inhibitor, 10 g/mL pepstatin, and 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride. The cells then were probe-sonicated (Sonifer Cell Disruptor, Heat Systems Co., Melville, NY, USA). The solubilized lysates were clarified by centrifugation at 8,000 ×g for 10 minutes, precleared with agarose, and then incubated with specific antibodies and protein-A agarose. The immunoprecipitates were collected and washed four times with lysis buffer. After heating at 95℃ for 5 minutes, the samples were centrifuged briefly, and the supernatants were analyzed by SDS-PAGE on 8% to 16% gradient gels.
Transiently transfected HIRcB cells were grown on glass-bottom microwell dishes (Mat Tek Corp., Ashland, MA, USA) for 24 hours. The cells were then placed in phenol red-free DMEM supplemented with 0.5% fetal bovine serum for 3 to 4 hours prior to experiments. The microwells were placed on a Zeiss Axiovert 135 confocal microscope (Zeiss Inc., Thornwood, NY, USA), and the cells were treated with insulin (100 ng/mL) at room temperature. Detection of GFP was carried out by recording at a wavelength of 488 or 568 nm, respectively.
Phosphotyrosine-mediated signal pathways have been shown to play an important role in insulin signaling. Tubby does not share any regions or domains of homology with other proteins, but Tub does have several potential tyrosine phosphorylation sites on its carboxyl-terminus. To investigate whether Tub becomes phosphorylated on tyrosine residue in response to insulin, we transfected Myc-tubby into HIRcB cells. Following treatment with insulin, immunoprecipitation with d-myc was conducted. Analysis of the whole cell lysate (WCL) by tyrosine-specific phosphorylation antibody (a-Ty[p]) immunoblot demonstrated that Tub was phosphorylated in a time/dose-dependent manner following insulin introduction (Fig. 1). Anti-Tub immunoblotting of the WCL demonstrated equivalent amounts of Tub expressed under the different conditions. These results indicate that insulin induces rapid and transient tyrosine phosphorylation of Tub at physiological doses.
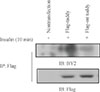
Phosphotyrosine-mediated interactions with SH2-containing signaling proteins have been shown to play an important role in the insulin pathway. Strikingly, several tyrosine residues are conserved in all tubby family members. Among these, a sequence surrounding tyrosine 464 (YIVM) fits the consensus motif YXXM for phosphorylation by insulin. To determine the possibility of whether this sequence may serve as potential site of phosphorylation by insulin, we constructed a point mt by replacing tyrosine 464 residue with alanine. To investigate whether Tub becomes phosphorylated on tyrosine residue 464 in response to insulin, we transfected wild-type (wt) flag-Tub and mt flag-Tub into HIRcB cells. Following treatment with insulin, immunoprecipitation with a-flag was performed. Analysis of the WCL by tyrosine-specific phosphorylation antibody immunoblot demonstrated that wt Tub was phosphorylated following insulin, but mt Tub was not (Fig. 2). Anti-tubby immunoblotting of the WCL demonstrated equivalent amounts of tubby expressed under the different conditions. These results demonstrated that tyrosine residue 464 of Tub was specifically phosphorylated by insulin.
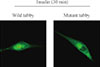
To analyze the insulin-dependent changes in an intracellular Tub location, GFP-tagged tubby proteins were constructed. HIRcB cells then were transiently transfected with the GFP-tubby. In the absence of insulin, GFP-tubby was uniformly distributed throughout the cytoplasm or plasma membrane with no fluorescence detectable in the nucleus. Analysis of the transfected cells showed that cells expressing GFP were cytoplasmic. Following the addition of insulin, however, substantial amounts of fluorescence appeared in the nucleus (Fig. 3). This result demonstrated that tubby translocated into the nucleus when activated by insulin and suggests that phosphorylation of tyrosine residue may be important in this process.
Since insulin activates the tyrosine phosphorylation of Tub at residue 464, we used insulin to test whether phosphorylation of this residue is important for nuclear translocation. The mt and wt constructs were then transiently expressed in HIRcB cells, and their translocation capacity in response to insulin was evaluated. Following the addition of insulin, cells transfected with the mt GFP-tubby showed substantial amounts of fluorescence in the nucleus (Fig. 4). Analysis of cells transfected with the mt GFP-tubby showed cytoplasmic expressed GFP. This result suggests that tyrosine residue 464 is important for nuclear translocation and tyrosine phosphorylation by insulin.
The major finding of this report is that tyrosine residue 464 of Tub becomes phosphorylated by insulin. Also, the data demonstrated that this tyrosine phosphorylation plays an important role in nuclear translocation of tubby. Therefore, tubby may be involved in insulin signaling.
The central question about the mechanism of nuclear translocation of tubby is determination of the driving force. The dual location of tubby in the plasma membrane and nucleus is indicative of a potential role in signal transduction between these two subcellular compartments. The dependence of membrane binding on specific phosphatidylinositol lipids indicates several potential pathways that might trigger tubby translocation to the nucleus. It is known that the Gaq/11 signal cascade [11], which functions downstream of Gaq/11, coupled seven transmembrane receptors. Activation of PLC, which is often regulated by Gaq, was shown to be required for nuclear translocation of tubby, as PLC inhibitor blocks translocation [12,13]. However, activation of Gaq might have a specific role in translocation other than the activation of PLC because overexpression of PLC is not sufficient for translocation [14,15]. The translocation mechanism of Tub thus remains unclear. Our study showed that tubby translocated into the nucleus with insulin. These data indicate that a mechanism other than G-protein-mediated signaling may be involved in tubby translocation.
The striking feature of the Tub family of proteins is a highly conserved C-terminus. Specifically, the last 250 amino acids are 55% to 95% identical across species, suggesting that this domain performs a very key function. Tub encodes a highly hydrophilic protein containing putative tyrosine phosphorylation and SH2-docking sites [16]. Phosphotyrosine-mediated interactions with SH2-containing signaling proteins have been shown to play an important role in intracellular signal transduction. Pre-incubation of the SH3 domain with phenylphosphate abolished its association with Tub, indicating that the association of Tub with SH2 domains is mediated through the interaction of the phosphotyrosine-binding pocket in the SH2 domains with specific phosphotyrosine-containing sequences on Tub. Also, several tyrosine residues are conserved in all Tub family members. Among these, the sequence surrounding tyrosine 464 (YIVM) fits the consensus motif YXXM for phosphorylation by insulin. The present study demonstrated that tyrosine residue 464 of Tub was specifically phosphorylated by insulin.
The aim of this study was to reveal the mechanism of tubby as nuclear localization signal. Although many aspects of tubby function have become clear, in particular, its role in diabetes and obesity, there are still several questions that remain regarding its complete function. Some future directions for research relating to tubby proteins are clear, and several issues hold exciting possibilities including target genes of tubby. In conclusion, the complete involvement of tubby in insulin signaling remains uncertain. It was shown that Tub is tyrosine-phosphorylated in response to insulin and insulin-like growth factor 1 but not by treatment with EGF. In vitro assay demonstrated that Tub was a target for phosphorylation by the insulin receptor and by JAK2. These data indicate that Tub might have a role in insulin signaling, and that Tub has the potential to function as an integrator of insulin and G-protein coupled receptor (GPCR)-mediated signals.
Figures and Tables
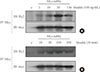 | Fig. 1Tubby gene (Tub) is phosphorylated by insulin. Phosphorylation of Tub by insulin. (A, B) HIRcB cells were transiently transfected with c-Myc-tubby. Following starvation, insulin was added. Immunoprecipitation (IP) reaction with anti-Myc antibody (Ab) and Western blot with phosphotyrosine Ab were performed to determine the phosphorylation of Tub. The lower panel is a Western blot demonstrating equivalent loading of total tubby in whole cell lysates used for the IP, using the tubby Ab. IB, immunoblotting. |
 | Fig. 2Tyrosine residue 464 of tubby gene (Tub) is an important phosphorylation site activated by insulin. Phosphorylation of Tub by insulin. HIRcB cells were transiently transfected with wild-type-flag-tubby and mutant-flag-tubby. Following starvation, insulin was added. Immunoprecipitation (IP) reaction with antiflag antibody (Ab) and Western blot with phosphotyrosine Ab were performed to determine the phosphorylation of Tub. The lower panel is a Western blot demonstrating equivalent loading of total tubby in whole cell lysates used for the IP, using the tubby Ab. |
 | Fig. 3Translocation of green fluorescent protein (GFP)-tubby in response to insulin in HIRcB cells. Serum-starved, transiently transfected HIRcB cells were used. Following starvation, insulin was added. After 30 minutes, the cellular location of GFP-tubby was observed using a confocal microscope with objective ×20. mt, mutant; IP, immunoprecipitation; IB, immunoblotting. |
 | Fig. 4Phosphorylation of tyrosine residue 464 of tubby gene (Tub) is important for nuclear translocation. Serum-starved HIRcB cells were used after transfection with wild-type green fluorescent protein (GFP)-tubby and mutant-GFP-tubby. Following starvation, insulin was added. After 30 minutes, the cellular locations of wild-type and mutant GFP-tubby were observed using a confocal microscope with objective ×20. |
ACKNOWLEDGMENTS
This study was supported by a Korea University Grant (K-1220321) and also supported by the Kil Chung Hee Fellowship Fund.
References
1. Carroll K, Gomez C, Shapiro L. Tubby proteins: the plot thickens. Nat Rev Mol Cell Biol. 2004; 5:55–63.
2. Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003; 421:268–272.
3. Ronshaugen M, McGinnis N, Inglis D, Chou D, Zhao J, McGinnis W. Structure and expression patterns of Drosophila TULP and TUSP, members of the tubby-like gene family. Mech Dev. 2002; 117:209–215.
4. Ikeda A, Naggert JK, Nishina PM. Genetic modification of retinal degeneration in tubby mice. Exp Eye Res. 2002; 74:455–461.
5. Ikeda A, Zheng QY, Zuberi AR, Johnson KR, Naggert JK, Nishina PM. Microtubule-associated protein 1A is a modifier of tubby hearing (moth1). Nat Genet. 2002; 30:401–405.
6. Grant SG. Putting tubby on the MAP. Nat Genet. 2002; 30:347–348.
7. Ikeda A, Nishina PM, Naggert JK. The tubby-like proteins, a family with roles in neuronal development and function. J Cell Sci. 2002; 115(Pt 1):9–14.
8. Li QZ, Wang CY, Shi JD, Ruan QG, Eckenrode S, Davoodi-Semiromi A, Kukar T, Gu Y, Lian W, Wu D, She JX. Molecular cloning and characterization of the mouse and human TUSP gene, a novel member of the tubby superfamily. Gene. 2001; 273:275–284.
9. Heikenwalder MF, Koritschoner NP, Pajer P, Chaboissier MC, Kurz SM, Briegel KJ, Bartunek P, Zenke M. Molecular cloning, expression and regulation of the avian tubby-like protein 1 (tulp1) gene. Gene. 2001; 273:131–139.
10. Hagstrom SA, Adamian M, Scimeca M, Pawlyk BS, Yue G, Li T. A role for the Tubby-like protein 1 in rhodopsin transport. Invest Ophthalmol Vis Sci. 2001; 42:1955–1962.
11. Koritschoner NP, Alvarez-Dolado M, Kurz SM, Heikenwalder MF, Hacker C, Vogel F, Munoz A, Zenke M. Thyroid hormone regulates the obesity gene tub. EMBO Rep. 2001; 2:499–504.
12. Cantley LC. Transcription. Translocating tubby. Science. 2001; 292:2019–2021.
13. Ikeda A, Ikeda S, Gridley T, Nishina PM, Naggert JK. Neural tube defects and neuroepithelial cell death in Tulp3 knockout mice. Hum Mol Genet. 2001; 10:1325–1334.
14. Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L. G-protein signaling through tubby proteins. Science. 2001; 292:2041–2050.
15. He W, Ikeda S, Bronson RT, Yan G, Nishina PM, North MA, Naggert JK. GFP-tagged expression and immunohistochemical studies to determine the subcellular localization of the tubby gene family members. Brain Res Mol Brain Res. 2000; 81:109–117.
16. Kapeller R, Moriarty A, Strauss A, Stubdal H, Theriault K, Siebert E, Chickering T, Morgenstern JP, Tartaglia LA, Lillie J. Tyrosine phosphorylation of tub and its association with Src homology 2 domain-containing proteins implicate tub in intracellular signaling by insulin. J Biol Chem. 1999; 274:24980–24986.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download