Abstract
Atypical antipsychotics have replaced conventional antipsychotics in the treatment of schizophrenia because they have less of a propensity to cause undesirable neurologic adverse events including extrapyramidal symptoms, tardive dyskinesia, and neuroleptic malignant syndrome (NMS). However, atypical antipsychotics have been known to result in various metabolic complications such as impaired glucose tolerance, diabetes and even diabetic ketoacidosis (DKA). In addition, a number of NMS cases have been reported in patients treated with atypical antipsychotics, although the absolute incidence of neurologic side effects is currently significantly low. Here, we report a patient who simultaneously developed DKA, acute renal failure and NMS with rhabdomyolysis after olanzapine treatment. Olanzapine-induced metabolic complications and NMS were dramatically improved with cessation of the olanzapine treatment and initiation of supportive management including fluid therapy, hemodialysis, and intensive glycemic control using insulin. At short-term follow-up, insulin secretion was markedly recovered as evidenced by a restoration of serum C-peptide level, and the patient no longer required any hypoglycemic medications. Despite the dramatic increase in the use of atypical antipsychotics treatment, individualized treatments along with careful monitoring may be prudent for high risk or vulnerable patients in order to avoid the development of metabolic side effects.
Atypical antipsychotics are associated with fewer neurological side effects such as extrapyramidal symptoms and tardive dyskinesia, which are the general side effects of conventional antipsychotics, but they are associated with increased risk of metabolic diseases including impaired glucose tolerance and diabetes. In addition, in spite of the reduced incidence of neurological side effects with atypical antipsychotics, acute neurological side effects including neuroleptic malignant syndrome (NMS) have been reported [1]. The atypical antipsychotics, olanzapine, and clozapine, are associated with a higher frequency of metabolic adverse effects, which can cause serious complications such as diabetic ketoacidosis (DKA) [2]. So far, there have only been a few individual case reports for DKA or NMS caused by atypical antipsychotics, but there have not been any reports on the simultaneous occurrence of both. Here we provide a literature review along with a case report of simultaneous occurrence of DKA and NMS in a patient who received long-term treatment with olanzapine.
A 29-year-old man presented at a emergency room in a stupor.
The patient had started psychiatric treatment after being diagnosed with personality disorder and hypochondriasis by the department of psychiatry in our hospital 4 years ago. After about 1 year of treatment, olanzapine was administered due to the patient's symptoms of social withdrawal and distrust of people. After the initiation of olanzapine, the patient's body weight increased by ≥30 kg, and hyperthermia occurred together with nausea and vomiting 1 week prior to the visit to our hospital. The patient received a prescription from a neighboring hospital and had taken antipyretics, but did not show any improvement. Then the patient showed mental deterioration and was admitted to our hospital via the emergency room.
Both parents were moderately built, and none of the family members, including siblings, had a metabolic disease such as diabetes.
The patient was on medication due to incidences of personality disorder, hypochondriasis, and psychotic tendency, taking olanzapine 10 mg, valproic acid 500 mg, clonazepam 0.5 mg, and lorazepam 1 mg on a daily basis. The aforementioned medication regimen was maintained for over 1 year without any dosage change, and the intake period of olanzapine was 32 months. There was no other underlying disease than the aforementioned, and there were no abnormal test results based on blood assay, including an assay for fasting plasma glucose, which was performed prior to administration of antipsychotics.
The patient had no history of smoking or alcohol drinking and was unemployed since completion of military service as social service personnel.
In physical examination, the patient showed acute signs of illness and was in a coma. His vital signs measured at the time of visit to our hospital were 110/70 mm Hg for blood pressure (BP), 120 beats per minute for pulse rate, 24 times per minute for respiratory rate, and 37.2℃ for body temperature. The patient's height was 181 cm, weight was 104 kg and body mass index (BMI) was 31.7 kg/m2. His consciousness state was stupor with normal light reflexes, and senses as well as spontaneous motions of extremities were maintained. Chest auscultation revealed that the heart rate was fast, but there was no murmur, and the patient had tachypnea, but had no crackles or wheezing sound auscultated. There was neither notable tenderness nor rebound tenderness from the abdomen. The patient's skin was dry overall.
Peripheral blood assay showed the following results: white blood cell 6,560/mm3 (neutrophil 72%), hemoglobin 20.2 g/dL, hematocrit 60.8%, and platelets 228,000/mm3. Taking the findings of physical examination into account, dehydration-induced hemoconcentration was suspected. The results of serum biochemical assay revealed that the plasma glucose level had increased to 1,216 mg/dL, the blood urea nitrogen level was 31 mg/dL, and creatinine level was 2.4 mg/dL. The result also showed the following: sodium, 149 mEq/L; potassium, 4.5 mEq/L; chloride, 96 mEq/L; total protein, 8.7 g/dL; albumin, 5.0 mg/dL; aspartate aminotransferase, 28 IU/L; alanine aminotransferase, 44 IU/L; total bilirubin, 0.6 mg/dL; calcium, 11.5 mg/dL; phosphate, 6.1 mg/dL; and creatine phosphokinase (CPK), 80 U/L (reference range, 30 to 170). Dipstick-utilized urine assay showed a positive reaction to ketone, and increases in amylase and lipase to 545 IU/L (reference range, 28 to 100) and 1,435 U/L (reference range, 13 to 60), respectively. Arterial blood gas analysis (ABGA) showed that the pH was 7.25, pCO2 was 18.9 mm Hg, pO2 was 86.1 mm Hg, HCO3- was 8.2 mEq/L, and oxygen saturation was 95.7%, presenting metabolic acidosis with 44.8 mEq/L of anion gap. The glycosylated hemoglobin level was 13.8%, while the serum C-peptide level tested during the administration of insulin were 0.38 ng/mL. So the patient was presumed to be in DKA and acute renal failure.
Electrocardiography findings showed sinus tachycardia with rate of 150 beats per minute.
There were no specific findings from the chest X-ray examination, and there was no evidence of ileus other than fecal impaction shown on abdominal X-ray examination. An abdominal computed tomography scan showed no findings of edema or necrosis or fluid retention in the pancreatic parenchyme and its periphery (Fig. 1).
Although there was no sign of BP reduction, the patient showed severe dehydration induced by extreme hyperglycemia and metabolic acidosis. We administered ≥4 L per day of fluid through intravenous infusion and immediately began insulin pump therapy. Within 24 hours of insulin therapy, the plasma glucose level was reduced to less than 300 mg/dL (Fig. 2), the anion gap level was reduced to normal range and ABGA showed that the patient was out of metabolic acidosis. But, in spite of fluid infusion of ≥2 L per day, hyperthermia occurred and the levels of CPK and creatinine began to increase rapidly to 16,161 U/L and 4.1 mg/dL, respectively, on the 3rd day of hospitalization. Also, the urinary myoglobin level was 1,879 ng/mL. In addition, the patient was in a confused mental state with severe muscle stiffness and rigidity, and increased uptake at upper and lower proximal limb was shown on bone scan (Fig. 3). Even with fluid treatment for acute renal failure, the level of creatinine continued to increase, so we started continuous renal replacement therapy (CRRT) on the 5th day of hospitalization (Fig. 4). After initiation of CRRT, the patient showed remarkable improvement in fever and consciousness, so we converted dialysis modality to intermittent hemodialysis 3 days later and maintained the treatment until the 12th day of hospitalization. The urine output was normalized and there was no additional elevation in creatinine and CPK concentrations, so we discontinued hemodialysis (Fig. 4). The patient presented consciousness deterioration, muscle stiffness, and high fever that did not respond to antipyretics and fluid infusion with history of taking antipsychotics, so we determined that the patient had NMS, and implemented supportive therapy including discontinuation of causative medication. We used the insulin pump for glycemic control in the early stage, and changed to multiple daily injections of insulin to manage the patient's condition. We could discontinue the administration of insulin on the 41st day after the first visit to our hospital as the insulin demand had gradually decreased. And we changed to metformin monotherapy. We stopped the administration of oral hypoglycemic agents on the 52nd day. On follow-up examination, we confirmed a notable reduction in glycosylated hemoglobin and a remarkable restoration of the serum C-peptide level (Table 1). Currently, the patient is maintaining very good glycemic control only by life style modification and is under prognostic observation as an outpatient.
Atypical antipsychotics have fewer neurological side effects such as extrapyramidal symptoms, akathisia, acute dystonia, NMS, and tardive dyskinesia, which are the weaknesses of conventional antipsychotics. Reduction of such neurological side effects is thought to be due to the diversity of target receptor of atypical antipsychotics, which have an affinity for various other receptors including serotonin, histamine, glutamine, and adrenergic receptors as well as dopamine receptor, in contrast to conventional antipsychotics, which act exclusively on the dopamine receptor, resulting in neurological vulnerability [3].
Atypical antipsychotics have become a mainstay of treatment for schizophrenia owing to the decrease in neurological side effects, but the fact that a risk of metabolic disease such as obesity, glucose intolerance and diabetes could rise became known. Fertig et al. [4] first proposed the association between olanzapine and abnormal glucose metabolism in 1998, and then Wilson et al. [5] reported that atypical antipsychotics-related DKA in 2003. In Korea, there was a report on changes in body weight, BMI, leptin, and fasting plasma glucose in a patient with schizophrenia who had received olanzapine in 2001, and the first cases of DKA caused by olanzapine and clozapine were reported in 2005 and 2007, respectively [1,6,7]. As a result of serious olanzapine-related metabolic complications, some countries have prohibited the use of olanzapine in patients with a past history of diabetes [8].
Meanwhile, although atypical antipsychotics have brought a notable reduction in neurological side effects such as extrapyramidal symptoms, in a few cases, the incidence of neurological side effects such as NMS continue to be reported, and therefore require clinical monitoring. NMS is a major adverse event in the treatment of psychosis, presenting as instability of autonomic nervous system with hyperthermia, extrapyramidal symptoms such as muscle rigidity, and changes in consciousness such as confusion and coma [9]. Although the cause of these adverse events has not been identified, there have been several hypotheses, such as blocking of the dopamine receptor via the nigrostriatal pathway inhibits the thermoregulation center and causes extrapyramidal symptoms; there is a defect of skeletal muscle similar to malignant hyperthermia; or neuroleptics cause muscular hypermetabolism [10-12]. Such functional failure of the thermoregulation center causes peripheral vasoconstriction, suppressing heat dissipation and inducing hyperthermia. The risk factors of NMS are dehydration, rapid incremental medication, past history of NMS, intravenous administration of medication, stress and infection, and olanzapine can also cause NMS [9,13,14]. Olanzapine demonstrates incomplete blocking of the D2 receptor and has a different blocking mechanism for 5-HT2 and the muscarinic receptor. Thus, it causes fewer extrapyramidal symptoms, which are the side effects of conventional antipsychotics, and fewer side effects related to blood, which is a weakness of clozapine. Nonetheless, although they are rare, acute neurological side effects have been reported, and they may occur because the serotonin receptor and noradrenalin receptor as well as the dopamine receptor can contribute to the incidence of NMS [10]. In this case, we determined that there was an association with the medication, so we discontinued the drug administration and began intensive glycemic control through the use of fluids and insulin, and then performed hemodialysis for rhabdomyolysis-induced acute renal failure. After these treatments, the patient showed notable recovery in terms of levels of plasma glucose, anion gap, creatinine and CPK concentrations, as well as clinical symptoms. The serum C-peptide was normalized within about 5 weeks, and when we discontinued the insulin treatment and changed to metformin monotherapy, the patient showed very good glycemic control. In this study, rhabdomyolysis-induced acute renal failure occurred without any notable evidence of trauma or infection during a long-term intake of atypical antipsychotics, so we initially suspected that the drug treatment was the cause. We identified the history of olanzapine intake, and considered the possibility that atypical antipsychotics were causing the DKA. Moreover, we predicted that the hyperthermia that was nonresponsive to antipyretics and the change in consciousness were also symptoms of NMS induced by olanzapine and the extremely stressful condition of DKA might exacerbate neurological side effects. Also, the patient had dramatic weight gain since the intake of olanzapine and DKA occurred with the increase in insulin resistance and decrease in insulin secretion. Such metabolic disturbance induce severe dehydration, and acute renal failure with rhabdomyolysis, and these conditions became risk factor for neurological side effects such as NMS.
After the supportive treatment involving discontinuation of medication and intensive glycemic control, the metabolic parameters and clinical symptoms were restored in a short time, indicating that the effects of olanzapine-induced metabolic complications can be reversed. Therefore, extra precaution is necessary to monitor possible metabolic complications such as obesity, impaired glucose tolerance, and diabetes when administering atypical antipsychotics like olanzapine. With atypical antipsychotics, the incidence of neurological side effects should be monitored carefully, even if they are rare, and this would involve monitoring the CPK concentration in connection to the administration of atypical antipsychotics such as olanzapine [15].
Based on existing reports, the incidence of DKA after administration of atypical antipsychotic medication occurs mostly during the initial stage of medication administration, and there was a report that it had occurred at 5.8 weeks on average after initiation of administration. However, as in this case, there were some cases of long-term administration of ≥1 year, and Wehring et al. had reported deaths at 14.5, 25.5, and 59.5 months, respectively, due to DKA after administration of clozapine [16]. The patient in this case demonstrated a prominent increase in weight after administration of olanzapine (181 cm, 70 kg, and BMI 21.4 kg/m2 to 104 kg and BMI 31.7 kg/m2) and the insulin resistance could contribute largely to the abnormal glucose metabolism. In particular, it is known that an increase in visceral fat accumulation contributes prominently to insulin resistance [17]. In addition to an increase in insulin resistance with olanzapine, its relevance to the deterioration in insulin secretion by β-cells had been questioned. Given the higher incidence of DKA within a short time after administration, the severe impairment of glucose metabolism that cannot be explained by only insulin resistance and the increase in body weight, and the frequency of DKA being higher in type I diabetes, we infer that inhibition on pancreatic β-cell function in insulin secretion is related to the administration of atypical antipsychotics. The mechanism of olanzapine-induced deterioration of insulin secretion is still unclear, but it may involve impaired insulin secretion and failure in compensatory upregulation of β-cell function through the neuropancreatic axis [17-19]. In this case, after discontinuation of medicine, the plasma glucose level was notably improved and the serum C-peptide level was restored, but the decrease in body weight was not so significant, suggesting that the deterioration of insulin secretion activity by medication is an appropriate finding for etiological cause of worsened glucose metabolism. Among atypical antipsychotics currently in use, clozapine and olanzapine are known to have the highest risk of metabolic complications. Reports on metabolic complications are extremely rare for aripiprazole, amisulpride, risperidone, and ziprasidone. The patient in this case was taking ziprasidone and was undergoing follow-up with no further glycemic excursions. Therefore, given the fact that the demand for atypical antipsychotics is increasing, there should be requirements to perform individualized selection of antipsychotic medication for patients as well as clinical monitoring in order to determine the actual risks of metabolic complications.
In summary, the increasing use of atypical antipsychotic medication raises the concern for increased incidences of metabolic complications including excessive weight gain, impaired glucose tolerance and diabetes. The patient in this study had a normal BMI, but after taking the antipsychotic olanzapine, his weight increased markedly. Despite the effort to reduce the neurological side effects by using of atypical antipsychotics, we experienced a case of DKA and NMS developing with rhabdomyolysis and acute renal failure simultaneously. With intensive treatment including discontinuation of medication, fluid therapy, administration of insulin and hemodialysis, the adverse effects were reversed. In subsequent follow-up observation, the patient demonstrated a quick restoration in insulin secretion activity, and ultimately was maintaining a good glycemic control without administration of oral hypoglycemic agents or insulin. In this report, we have presented the details of this case, together with a literature review.
Figures and Tables
Fig. 1
Nonenhanced computed tomography showed no definite pancreatic parenchymal infiltration or abnormal fluid collection.
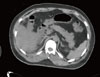
Fig. 2
Intensive glycemic control using insulin resulted in remarkably improved serum glucose levels. MDI, multiple dose injection.

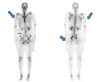
Fig. 3
Bone scan. Blue arrows indicated mild uptake at both the upper proximal and lateral thigh and buttock.
References
1. Kwak YT, Koo MS. Olanzapine-induced neuroletpic malignant syndrome. J Korean Neurol Assoc. 2000. 18:249–251.
2. Jeon YJ, Lee SH, Jang SN, Kim ES, Min JY, Kim JH, Hong SH, Cho JH, Kwon HS, Yoon KH, Cha BY, Son HY. Diabetic ketoacidosis in a patient with long-term clozapine therapy. J Korean Endocr Soc. 2007. 22:376–380.
3. Kinon BJ, Lieberman JA. Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacology (Berl). 1996. 124:2–34.
4. Fertig MK, Brooks VG, Shelton PS, English CW. Hyperglycemia associated with olanzapine. J Clin Psychiatry. 1998. 59:687–689.
5. Wilson DR, D'Souza L, Sarkar N, Newton M, Hammond C. New-onset diabetes and ketoacidosis with atypical antipsychotics. Schizophr Res. 2003. 59:1–6.
6. Kim YR, Kwon YJ, Park IJ, Jung HY, Woo KM, Cho MH. Body weight, body mass index, plasma leptin, insulin and fasting glucose level in schizophrenic patients receiving olanzapine. J Korean Neuropsychiatr Assoc. 2001. 40:1240–1246.
7. Lee JS, Kim JY, Ahn JH, Kim CY. Diabetic ketoacidosis in a schizophrenic patient treated with olanzapine: a case report. J Korean Neuropsychiatr Assoc. 2005. 44:116–119.
8. Misawa F, Miyaji S, Fuji Y, Miyata R, Obi K, Koshiishi F, Shimada H, Fukuda M, Koizumi T. The incidence of hyperglycemia in patients treated with olanzapine. J Clin Psychiatry. 2004. 65:443–444.
9. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993. 77:185–202.
10. Khaldi S, Kornreich C, Choubani Z, Gourevitch R. Neuroleptic malignant syndrome and atypical antipsychotics: a brief review. Encephale. 2008. 34:618–624.
11. Caroff S, Rosenberg H, Gerber JC. Neuroleptic malignant syndrome and malignant hyperthermia. J Clin Psychopharmacol. 1983. 3:120–121.
12. May DC, Morris SW, Stewart RM, Fenton BJ, Gaffney FA. Neuroleptic malignant syndrome: response to dantrolene sodium. Ann Intern Med. 1983. 98:183–184.
13. Filice GA, McDougall BC, Ercan-Fang N, Billington CJ. Neuroleptic malignant syndrome associated with olanzapine. Ann Pharmacother. 1998. 32:1158–1159.
14. Srivastava A, Borkar HA, Chandak S. Olanzapine-induced neuroleptic malignant syndrome in a patient with paranoid schizophrenia. Psychiatry Clin Neurosci. 2009. 63:119–121.
15. Baumgart U, Schmid R, Spiessl H. Olanzapine-induced acute rhabdomyolysis: a case report. Pharmacopsychiatry. 2005. 38:36–37.
16. Wehring HJ, Kelly DL, Love RC, Conley RR. Deaths from diabetic ketoacidosis after long-term clozapine treatment. Am J Psychiatry. 2003. 160:2241–2242.
17. Ader M, Kim SP, Catalano KJ, Ionut V, Hucking K, Richey JM, Kabir M, Bergman RN. Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease: a placebo-controlled study of olanzapine and risperidone in dogs. Diabetes. 2005. 54:862–871.
18. Hardy TA, Meyers AL, Yu J, Shankar SS, Steinberg HO, Porksen NK. Acute insulin response and beta-cell compensation in normal subjects treated with olanzapine or risperidone for 2 weeks. Diabetes Care. 2007. 30:157–158.
19. Sowell MO, Mukhopadhyay N, Cavazzoni P, Shankar S, Steinberg HO, Breier A, Beasley CM Jr, Dananberg J. Hyperglycemic clamp assessment of insulin secretory responses in normal subjects treated with olanzapine, risperidone, or placebo. J Clin Endocrinol Metab. 2002. 87:2918–2923.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download