Abstract
Cancer metastases to the thyroid or adrenal gland are uncommon. Furthermore, cases showing long-term survival after surgical resection of those metastatic tumors are rare. We report a case of pulmonary artery intimal sarcoma with metastases to the thyroid and adrenal glands sequentially that was successfully treated with sequential metastasectomies. A 62-year-old woman presented with a 4-week history of dyspnea on exertion and facial edema in November 1999. Echocardiography and chest computed tomography (CT) revealed an embolism-like mass in the pulmonary trunk. Pulmonary artery endarterectomy with pulmonary valve replacement was performed, and histopathology revealed pulmonary artery intimal sarcoma. A thyroid nodule was found by chest CT in November 2001 (2 years after initial surgery). During follow-up, this lesion showed no change, but we decided to obtain fine needle aspiration cytology (FNAC) in August 2004 (4.7 years after initial surgery). FNAC revealed atypical spindle cells suggestive of metastatic intimal sarcoma. She underwent total thyroidectomy. During follow-up, a right adrenal gland mass was detected by chest CT in March 2006 (6.3 years after initial surgery), and adrenalectomy was done, which also revealed metastatic sarcoma. She has been followed up without any evidence of recurrent disease until May 2012 (12.5 years after initial surgery).
Pulmonary artery sarcoma is an uncommon malignancy. It was first reported in 1923 in an autopsy case by Mandelstamm, and since then, fewer than 250 cases have been described [1,2]. The prognosis of patients with pulmonary artery sarcoma is usually poor. In reported cases, the mean survival time was usually less than 2 months for those without surgical treatment and approximately 10 months for those treated surgically [3]. Surgical resection, if possible, is known to be the best therapeutic option to prolong survival [4].
Thyroid and adrenal glands are representative endocrine organs that have high vascularity, and we often encounter thyroid or adrenal metastases in autopsy cases with malignant diseases; however, malignant metastasis to both endocrine organs during follow-up is not common in the clinical setting. As imaging techniques and fine needle aspiration skills improve, asymptomatic solitary metastases are more frequently discovered [5].
Resection of metastatic disease has been shown to prolong survival of patients in a variety of cancers. Metastasectomy can be justified in liver metastases from colorectal cancer or neuroendocrine tumors and in lung metastases from colorectal cancer or from osteogenic sarcoma. There has been a limited number of reports evaluating the role of thyroid and/or adrenal metastasectomy [6-10].
Here we report a case of a patient with pulmonary artery intimal sarcoma who showed long-term survival after sequential metastasectomies of the thyroid and adrenal glands.
A 62-year-old woman presented with a 4-week history of dyspnea on exertion and facial edema in November 1999. Her past medical history was unremarkable. Electrocardiography showed T wave inversion at leads V1-5, III, and aVF, right axis deviation, and right ventricular hypertrophy. Initial echocardiography revealed an echogenic mass in the main pulmonary artery with significant flow obstruction and right ventricular dysfunction suggesting pulmonary embolism. Chest computed tomography (CT) revealed an embolism-like mass in the pulmonary trunk and in both sides of the main pulmonary arteries (Fig. 1A). Pulmonary artery endarterectomy with pulmonary valve replacement and pulmonary artery reconstruction were performed. The surgical specimen showed an ill-defined, protruding polypoid mass that was 4.8 cm in the greatest dimension. The pathology revealed pulmonary artery intimal sarcoma, which was supported by positive smooth muscle actin and negative desmin in immunohistochemical staining (Fig. 1B). Involvement of the resection margin by the tumor was noted. She received radiation therapy (4,500 cGy fractionated) and chemotherapy (cyclophosphamide, doxorubicin, and dacarbazine) as adjuvant treatments. During follow-up, she was regularly checked by echocardiography and chest CT. There was no evidence of local recurrence on echocardiography. On initial follow-up chest CT (November 2001, 2 years after initial surgery), mild diffuse enlargement of the thyroid gland with multiple small low density lesions was discovered. It is hard to know if these findings existed before the initial operation, because the preoperative chest CT did not include the neck. She showed normal thyroid function tests. This lesion was just followed up with chest CT yearly and showed no change for 3 years.
In August 2004 (4.7 years after initial surgery), she was referred to an endocrinologist for abnormal thyroid function tests. There was a palpable nodule in the left thyroid gland. Cervical lymphadenopathy was not present. The serum thyroid stimulating hormone level was 0.12 mIU/L (normal, 0.4 to 5.0), and total T4 was 166 nmol/L (normal, 76 to 178). CT of the neck showed two low density nodules of 2.0×2.5 cm and 0.9×0.9 cm in both lobes of the thyroid glands, which was no different from previous studies (Fig. 2A). Fine needle aspiration cytology was done and revealed atypical spindle cells, suggestive of metastatic intimal sarcoma (Fig. 2B). She had no other metastatic sites in the chest and abdomen-pelvic CT. She underwent total thyroidectomy, and histology confirmed metastatic intimal sarcoma with clear resection margins. She was followed by neck and chest CT (covering the adrenal gland), and there was no evidence of local recurrence or distant metastasis until August 2005.
A 10.0×8.5 cm sized round, heterogeneous mass replacing the whole adrenal gland superior to the right kidney was newly detected by chest CT in March 2006 (6.3 years after initial surgery), suggesting a metastatic lesion in the right adrenal gland (Fig. 3A). An 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET)/CT scan showed a large hypermetabolic mass (max standardized uptake value, 6.8) with internal necrosis (Fig. 3B). The lesion was suspected as a recurrence of intimal sarcoma. Right adrenalectomy was done without preoperative biopsy. The histopathologic diagnosis was metastatic intimal sarcoma. The tumor cells were positive for smooth muscle actin and were negative for inhibin, cytokeratin, chromogranin, and desmin, supporting the above diagnosis.
During follow-up after right adrenalectomy, abdomen-pelvic CT and 18F-FDG PET/CT showed no evidence of recurrences or metastases, including the anastomosis site of the main pulmonary trunk, thyroid bed, and adrenalectomy site. She has been alive and well without any evidence of disease as of May 2012 (12.5 years after initial surgery).
Intimal sarcoma is a rare tumor that arises from large vessels, including the aorta and pulmonary artery. It is characterized by insidious growth, but it usually presents with extensive local invasion and hematogenous metastases at the time of diagnosis. Metastases are most commonly found in the lungs, although deposits in the pancreas, kidney, brain, lymph nodes, and skin have been reported [11]. The prognoses of those patients are usually poor. Most patients die within months of the initial presentation. The main treatment of choice is radical surgical resection [1,4].
Although endocrine organs have high vascularity, metastasis to endocrine organs in the clinical setting is not as common; however, recent autopsy studies have shown that the incidence of thyroid metastases ranges between 1% and 24% in patients with a known history of neoplasm [12]. The prevalence of adrenal metastases in patients with extra-adrenal cancers ranges from 32% to 73% in different series [13].
Breast cancer and lung cancer are the most frequent malignancies showing metastases to the thyroid. In the case of adrenal metastasis, lung, kidney, breast, and gastrointestinal carcinomas are the most frequent primary malignancies [14]. As ultrasonography-guided aspiration and various imaging modalities such as 18F-FDG PET/CT have been more frequently used in clinical settings, more cases with metastases to the thyroid or adrenal gland are encountered. Moreover, asymptomatic solitary metastatic lesions are more likely to be found before they become widespread. Thus, a thyroid or adrenal mass in a patient with a history of a previous malignancy should be regarded as potentially metastatic, even if the primary tumor was adequately treated many years before [15,16].
No standard therapeutic guidelines have been established for solitary metastatic disease to the thyroid or adrenal gland. The efficacy of surgical treatment for a solitary metastasis as seen in our case is unknown. Numerous case reports have suggested that metastases to the thyroid or adrenal gland are associated with a poor prognosis, because most thyroid or adrenal metastases occur late in the course of disseminated cancers [15,17]. Surgical treatment for metastases has not been widely adopted because of the concern about probable associated advanced diffuse metastatic diseases; however, radical treatment for an isolated metastasis to the thyroid or adrenal gland can be curative or achieve long-term survival, and an aggressive surgical approach has been recommended by some authors [6-10,18]. Surgery has been advocated for metastatic disease, provided the disease is limited and/or amenable to complete treatment. The therapeutic aim should be with a curative or complete intent. Preoperative investigations should be as extensive as possible in order to detect any inaccessible metastatic sites or invasion hampering complete surgery [19,20]. The prognosis of patients who have a solitary thyroid or adrenal metastasis depends on multiple factors such as the site of origin, the histological cell type of primary tumors, antecedent history of distant metastasis or discovery of other synchronous distant metastasis, and extent of local disease [9]. Thus, careful patient selection is extremely important. The primary tumor should be controlled. There should be no extra thyroid or adrenal disease, and preoperative imaging studies should demonstrate disease that is likely to be completely resectable. Furthermore, as in our case, an additional metastatic site does not have to be an exclusion criterion if it seems to be curable.
We treated a rare case with intimal sarcoma of the pulmonary artery that metastasized to the thyroid and adrenal glands sequentially. The patient developed a thyroid metastasis 4.7 years after initial surgery and an adrenal metastasis 6.3 years after initial surgery. Both metastases were successfully controlled by surgical resections. The patient showed unusual long-term survival up to 12.5 years. There should be more investigations regarding which cancers should be considered for metastasectomy of the endocrine organs with curative intent.
References
1. Xu Y, Wang K, Geng Y, Shao Y, Yin Y. A case of intimal sarcoma of the pulmonary artery successfully treated with chemotherapy. Int J Clin Oncol. 2012; 17:522–527. PMID: 22041929.

2. Blackmon SH, Rice DC, Correa AM, Mehran R, Putnam JB, Smythe WR, Walkes JC, Walsh GL, Moran C, Singh H, Vaporciyan AA, Reardon M. Management of primary pulmonary artery sarcomas. Ann Thorac Surg. 2009; 87:977–984. PMID: 19231448.

3. Jin T, Zhang C, Feng Z, Ni Y. Primary pulmonary artery sarcoma. Interact Cardiovasc Thorac Surg. 2008; 7:722–724. PMID: 18467425.

4. Said SM, Sundt TM 3rd, Garces YI, Wigle DA. 5-year survival after multiple repeat metastasectomy for pulmonary artery angiosarcoma. Ann Thorac Surg. 2011; 91:e49–e51. PMID: 21440106.

5. Kim TY, Kim WB, Gong G, Hong SJ, Shong YK. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clin Endocrinol (Oxf). 2005; 62:236–241. PMID: 15670202.

6. Ayabe H, Tsuji H, Hara S, Tagawa Y, Kawahara K, Tomita M. Surgical management of adrenal metastasis from bronchogenic carcinoma. J Surg Oncol. 1995; 58:149–154. PMID: 7898109.

7. Ericsson M, Biorklund A, Cederquist E, Ingemansson S, Akerman M. Surgical treatment of metastatic disease in the thyroid gland. J Surg Oncol. 1981; 17:15–23. PMID: 7230827.

8. Kim SH, Brennan MF, Russo P, Burt ME, Coit DG. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer. 1998; 82:389–394. PMID: 9445197.

9. Lo CY, van Heerden JA, Soreide JA, Grant CS, Thompson GB, Lloyd RV, Harmsen WS. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg. 1996; 83:528–531. PMID: 8665251.

10. Rosen IB, Walfish PG, Bain J, Bedard YC. Secondary malignancy of the thyroid gland and its management. Ann Surg Oncol. 1995; 2:252–256. PMID: 7641022.

11. Rashid A, Molloy S, Lehovsky J, Tirabosco R, Hughes R, Butt S. Metastatic pulmonary intimal sarcoma presenting as cauda equina syndrome: first report of a case. Spine (Phila Pa 1976). 2008; 33:E516–E520. PMID: 18594450.
12. Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997; 79:574–578. PMID: 9028370.
13. Terzolo M, Bovio S, Pia A, Reimondo G, Angeli A. Management of adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab. 2009; 23:233–243. PMID: 19500766.

14. Young WF Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007; 356:601–610. PMID: 17287480.
15. Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf). 2002; 56:95–101. PMID: 11849252.

16. McCabe DP, Farrar WB, Petkov TM, Finkelmeier W, O'Dwyer P, James A. Clinical and pathologic correlations in disease metastatic to the thyroid gland. Am J Surg. 1985; 150:519–523. PMID: 4051119.

17. Lam KY, Lo CY. Metastatic tumors of the thyroid gland: a study of 79 cases in Chinese patients. Arch Pathol Lab Med. 1998; 122:37–41. PMID: 9448014.
18. Urschel JD, Finley RK, Takita H. Long-term survival after bilateral adrenalectomy for metastatic lung cancer: a case report. Chest. 1997; 112:848–850. PMID: 9315827.
19. Sebag F, Calzolari F, Harding J, Sierra M, Palazzo FF, Henry JF. Isolated adrenal metastasis: the role of laparoscopic surgery. World J Surg. 2006; 30:888–892. PMID: 16547618.

20. Abdalla EK, Pisters PW. Metastasectomy for limited metastases from soft tissue sarcoma. Curr Treat Options Oncol. 2002; 3:497–505. PMID: 12392639.

Fig. 1
Chest computed tomography (CT) at initial diagnosis and final pathology after initial surgery. (A) Chest CT revealed an embolism-like mass in the pulmonary trunk (arrow). (B) Pathology showed the typical whorl formation pattern of sarcomas (H&E stain, ×400).
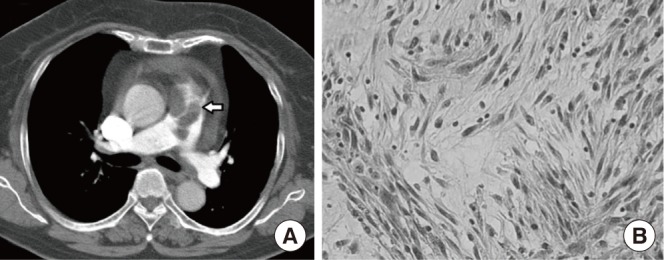
Fig. 2
Neck computed tomography (CT) and fine needle aspiration (FNA) smear results of the thyroid nodule. (A) Neck CT showed enlarged thyroid glands with a low density nodule in the left thyroid gland (2.0×2.5 cm) (arrow). (B) The FNA smear revealed atypical spindle cells, suggestive of metastatic intimal sarcoma (Papanicolaou stain, ×400).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download