Abstract
Due to the increased prevalence of papillary thyroid carcinoma (PTC), difficult cases and unexpected events have become more common during long-term follow-up. Herein we reported four cases that exhibited poor progress during long-term follow-up. All the cases were diagnosed with PTC and treated with total thyroidectomy before several years, and the patients had been newly diagnosed with recurrent and metastatic PTC. These four cases included recurred PTC with invasion of large blood vessels, a concomitant second malignancy, malignant transformation, and refractoriness to treatment. Physicians should closely monitor patients to promptly address unforeseen circumstances during PTC follow-up, including PTC recurrence and metastasis. Furthermore, we suggest that the development of a management protocol for refractory or terminal PTC is also warranted.
The prevalence of papillary thyroid carcinoma (PTC) is approximately 5% of female population and 1% of male population in countries, like Korea, where iodine is sufficiently supplied. The prognosis of PTC is relatively better than other types of cancer, and the 5-year survival rate can be as high as 90% to 95% [1-3]. Thus, the cumulative number of patients with PTC is increasing annually and, as those accumulated patients are observed through long-term follow-up and treated. Accordingly, the frequency of patients with poor prognosis or aggravated disease is also increasing. Even though there are well-established standard therapeutic methods available for the follow-up and treatment of PTC, we experienced several cases that needed supplementary care options to the standard therapeutic methods [2]. Herein, the authors report these cases to remind some difficulties in the long term follow-up of patients with PTC.
A 74-year-old female patient visited the emergency center with respiratory distress, facial edema, and sore throat as her primary complaints. The patient had a surgical history of total thyroidectomy due to PTC accompanied by multiple lymph node metastases performed 18 years prior, and she had received postoperative high-dose radioactive iodine (RAI) ablation (150 mCi) at the time. Physical examination revealed a palpable mass on the patient's neck with pus discharge from a neck scar (Fig. 1A). On neck sonography, a tumor thrombus was found in the right internal jugular vein (Fig. 1B). On chest computed tomography (CT) and positron emission tomography (PET)-CT, a large tumor causing superior vena cava syndrome, a tumor in the right upper lobe of the lung and multiple perimediastinal lymph node enlargements were found (Fig. 1C). The authors performed a CT-guided percutaneous fine needle biopsy of an enlarged lymph node and the tumor in the right upper lobe of the lung to establish a histological diagnosis (Fig. 1D). The biopsy revealed metastatic PTC (Fig. 1E). We considered immediate surgical treatment for each tumor, but deferred surgery due to the patient's disease progression. Instead, we started the high-dose RAI therapy on the basis of antithrombotic therapy. Nevertheless, the patient exhibited symptoms such as headache, dyspnea, and hemoptysis just a week after initiation of the antithrombotic therapy, and, when we performed additional examination, we observed brain metastasis (Fig. 1F). Consequently, the patient discontinued antithrombotic therapy and continued conservative therapy. However, the patient expired after 2 weeks.
A 44-year-old female patient with a history of PTC was reoperated due to the PTC recurrence in a neck lymph node. The patient was diagnosed and treated 10 years prior with total thyroidectomy and high-dose RAI ablation (150 mCi). At that time, we performed the lymph node dissection in the neck as well as high-dose RAI ablation (200 mCi). During follow-up, we conducted neck sonography, measurement of thyroxin withdrawal thyroglobulin level, whole-body 131I scan, and PET-CT, but none of these tests revealed any findings of remnant PTC. However, after 1 year, follow-up chest X-ray indicated ~0.3 cm lung nodules in lung fields on both sides. The tentative diagnosis was that these were benign lesions according to the interpretation of the radiologist. Due to the patient's past history of thyroid cancer, however, we shortened the follow-up interval and took another chest CT 3 months later. The chest CT did not show any significant differences from the previous chest X-ray (Fig. 2A). When compared with chest CT images taken 3 months later, we were able to observe slight increases in the size of the lesions. We performed a biopsy of the lung nodes by video-assisted thoracoscopic surgery, which resulted in a diagnosis of metastatic papillary thyroid cancer and bronchioloalveolar carcinoma (Fig. 2B).
A 68-year-old female patient, who was diagnosed with PTC and underwent the total thyroidectomy 10 years prior, had a hypoechoic neck lymph node enlargement revealed on follow-up neck sonography (Fig. 3A). We performed a fine needle aspiration biopsy of the enlarged lymph node, and the biopsy results showed recurrent metastatic PTC. We planned immediate surgical intervention, however, in the process of preparing for the surgery, the patient complained of a mild degree of dyspnea accompanied by chest pain on the right side. When chest X-rays were taken, we observed pleural thickening and pleural fluid on the right side. We performed a pleural biopsy, and biopsy findings indicated metastasis of PTC. Two days later, the patient's dyspnea was even more aggravated, and we noted compression of trachea caused by mediastinal lymph node enlargement and a rapid increase in the amount of pleural fluid on subsequent chest CT (Fig. 3B). The patient died 3 weeks after the diagnosis of metastasis of PTC in spite of conservative palliative treatment. The cause of death was suggested to be the aggressive anaplastic change of PTC or aggressive malignancy of other organ including esophagus or lung.
A 67-year-old female patient received total thyroidectomy due to PTC. Metastases of the carcinoma in the neck lymph node were found, and the patient underwent lymph node dissection in the neck area as a secondary procedure (Fig. 4A). After all procedures were completed, she received high-dose RAI therapy. While conducting a 2-year follow-up, lung nodules were found, and we conducted additional high-dose RAI therapy. A post-RAI follow-up chest CT was conducted, but revealed an increase in the number and size of nodules (Fig. 4B). RAI ablation (200 mCi) was performed again due to refractory disease. The patient complained of pain in thoracic wall and dyspnea after 6 months, and chest CT findings showed a large amount of pleural fluid on the right side, as well as metastatic lymph nodes (Fig. 4C). We performed a pleural biopsy in addition to pleural fluid drainage and pleurodesis (Fig. 4D). Nevertheless, the amount of pleural fluid continued to increase and dyspnea was aggravated. Consequently, the condition of the patient deteriorated and the disease remained refractory to treatment, so we maintained only conservative treatment. The patient died 3 months after pleural fluid was first observed.
The 10-year survival rate of PTC patients is usually over ~80% to 95%; however, if it is accompanied by distant metastasis, the 10-year survival rate drops to ~24% [4]. However, the overall prognosis is relatively good; patients or their families tend to have high expectations for cure of PTC. Such expectations may make it difficult to understand that the prognosis can worsen over long-term follow-up, due to recurrence or metastases to other organs. In addition, in terms of treatment, PTC progresses slowly and its malignant degeneration is lower than other malignant tumors that develop in other organs, so that treatment is provided on the basis of surgical removal of PTC, high-dose RAI therapy and internal medicine therapy to maintain a low thyroid stimulating hormone level.
Recently, as the detection rate of thyroid nodules is on the rise, diagnosis of PTC is also increasing in a substantially younger age group. Young patients in particular have a longer life expectancy, so that the need for long-term follow-up becomes more prominent, requiring more accuracy and detailed precision in examinations for the recurrence and aggravation of cancer through follow-up.
To this end, the authors reported four cases whose PTC had poor prognosis. All of these patients had been diagnosed with PTC and received total thyroidectomy, but had shown findings of recurrence and metastases after substantial time had passed. These included patients who had recurrence of PTC in structures adjacent to the thyroid, concurrent development of other organ cancers, anaplastic transformation or dedifferentiation, and disease refractory to typically curative treatments. Therefore, clinicians should pay careful attention when performing follow-up in patients with PTC even if they have shown good prognosis.
The first case had findings of tumor thrombus in superior vena cava, compression of the trachea, superior vena cava syndrome, and metastasis of PTC to the brain that are considered to be the end stage findings of cancer metastases. These findings show serious and various substantial thyroid metastases, and as the development and progression of PTC itself would have been significantly slower than other types of cancer. When the follow-up examination was performed at adequate time, the complications of thyroid cancer metastases would be minimized than this case. Wada et al. [5] reported two very rare cases that exhibited PTC, wide-ranging tumor thrombi in the superior vena cava, and metastases to the adjacent region of the superior vena cava and to the mediastinal lymph node. Both patients underwent wide resection of the superior vena cava and reconstructive procedures sufficiently early. Brain metastasis of PTC is very rare, and its prognosis is quite poor, but several cases with relatively good prognoses have also been reported, as a result of early diagnosis and adequate treatment [6,7]. This case emphasize the importance of early detection in the treatment of recurrent PTC and its metastasis through periodic sonographic follow-up examination of structures typically adjacent to the thyroid, despite total thyroidectomy. The second case reported reconfirmed that thyroid carcinoma can metastasize to the lung and can be accompanied by primary lung cancer in 15% to 47% of PTC patients. Roscoe et al. [8] reported a case of PTC which metastasized to the right upper lobe of the lung in a patient who was diagnosed with cicatrical primary adenocarcinoma and presented with moderate differentiation on postpneumonectomy biopsy, with findings of metastatic PTC cells present in mixed multiplicity. Therefore, when performing long-term follow-up of patients with PTC, clinicians should be aware that metastasis to other organs is possible in addition to recurrence and metastasis of PTC, and lesions in other organs should not be overlooked no matter how small they are. In addition, although metastasis of cancer to cancer is quite rare, but in case of PTC, cautions should be made for the fact that metastasis to the primary lung cancer can be happened. The third case reported in the present study was a patient who was diagnosed with metastasis of PTC to the pleura accompanied by pleural fluid, who had died within 3 weeks of diagnosis. In this case, the prognosis of the patient did not become poorer due to the progression of PTC, which has relatively slow progression and low aggressiveness of malignancy. Therefore, the authors hypothesized that the patient in this case was likely to have experienced dedifferentiation of PTC into an aggressive tumor with rapid progression, or to be transformed into malignancy of other organ rather than the issue of PTC itself only. Ogawa et al. [9] observed degradation of PTC into highly malignant cancer cells. In this case, the patient was diagnosed with PTC and metastasis to the lung and had been treated by lymph node dissection and total thyroidectomy and had completed RAI therapy. Nevertheless, the patient died suddenly due to seeding of cancer cells. The fourth case demonstrates that it is possible for this disease to be refractory to treatment when there are findings of recurrence and metastasis of PTC at the pleura with accompanying pleural fluid. If the disease is refractory to treatment, the prognosis, of course, is not good. Therefore, in cases of showing signs of recurrence and metastasis of PTC, but demonstrating little or no response to adequate treatment, particularly in patients exhibiting symptoms of pleura metastasis accompanied by pleural fluid, preemptive and immediate treatment will be required, even if the current condition and prognosis of the patient appear to be good.
As the cases presented here, careful long-term follow-up should be undertaken even though thyroid cancer patients have a good prognosis. Furthermore, for patients who respond poorly to current therapeutic tools, more developed management modalities should be investigated.
In summary, PTC is known to have a good prognosis if treated properly at an early stage, however, we should keep in mind the possibility of facing the unexpected aggravated patients. To this end, the authors aimed to inform clinicians by presenting several rare cases representative of those likely to have poor prognoses during long-term follow-up in patients with PTC.
Figures and Tables
Fig. 1
(A) A palpable mass and protruding scar with discharge were observed on a patient's neck at admission. (B) Neck sonography revealed a metastatic lymph node (LN) and tumor thrombus in the right internal jugular vein; however, a recurred mass was not seen at the thyroid bed. (C) Chest computed tomography (CT) showed a large lung mass and fat infiltration around the mass. The superior vena cava was compressed by the mass. Hematogenous metastatic lung nodules in the right lung were also detected. (D) Follow-up chest CT showed noticeably increased lung mass. (E) Biopsy findings from the right supraclavicular lymph node (LN) were compatible with metastatic papillary thyroid carcinoma (H&E stain, ×100). (F) A metastatic brain lesion was detected incidentally on neck CT.
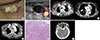
Fig. 2
(A) Chest computed tomography revealed two pulmonary nodules (arrows). The nodule located at the RLL was a ground glass opacity (GGO) pattern and the nodule at the LLL was a solid and GGO pattern. These were thought to be benign nodules by the radiologist. (B) H&E staining of metastatic papillary thyroid carcinoma within the bronchioloalveolar carcinoma (BAC). (Ba) The BAC contained a microscopic lesion showing papillary structures (arrows) (H&E stain, ×10). (Bb) The greater part of the nodule showed histological features typical of BAC (H&E stain, ×100). (Bc) At higher magnification of the papillary area, papillary structures lined by cells showed nuclear features of PTC (H&E stain, ×200).
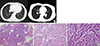
Fig. 3
(A) Follow-up neck sonography showed small nodules which were thought to be metastatic papillary thyroid carcinoma. (B) Follow-up chest computed tomography (CT) showed mediastinal lymph node enlargement and tracheal obstruction with pleural effusion that were not detected by previous chest CT. And also abdominal CT showed the adrenal metastatic mass (white arrow).

Fig. 4
(A) Neck sonography showed metastatic lymph nodes in the right lower jugular chain (left figure) and in the left supraclavicular area (right). (B) Follow-up chest computed tomography (CT) showed an enlarged nodule at the right minor fissure and a new small nodule at the RLL. (C) Follow-up chest CT after 6 months showed multiple metastatic lymphadenopathies, right pleural effusion with metastatic pleural mass, and hematogenous lung metastasis in the right lower lobe and middle lobe. (D) Cytologic finding of pleural fluid was compatible with metastatic papillary thyroid carcinoma (Pap stain, ×400).

References
1. Emerson CH. Guidelines for guidelines: content, accountability, peer review, and intellectual ownership. Thyroid. 2009; 19:1137–1138.
2. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.
3. Mallick UK. American Thyroid Association. The revised American Thyroid Association management guidelines 2009 for patients with differentiated thyroid cancer: an evidence-based risk-adapted approach. Clin Oncol (R Coll Radiol). 2010; 22:472–474.
4. Mazzaferri EL, Young RL, Oertel JE, Kemmerer WT, Page CP. Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine (Baltimore). 1977; 56:171–196.
5. Wada N, Masudo K, Hirakawa S, Woo T, Arai H, Suganuma N, Iwaki H, Yukawa N, Uchida K, Imoto K, Rino Y, Masuda M. Superior vena cava (SVC) reconstruction using autologous tissue in two cases of differentiated thyroid carcinoma presenting with SVC syndrome. World J Surg Oncol. 2009; 7:75.
6. Miranda ER, Padrao EL, Silva BC, De Marco L, Sarquis MS. Papillary thyroid carcinoma with brain metastases: an unusual 10-year-survival case. Thyroid. 2010; 20:657–661.
7. Al-Dhahri SF, Al-Amro AS, Al-Shakwer W, Terkawi AS. Cerebellar mass as a primary presentation of papillary thyroid carcinoma: case report and literature review. Head Neck Oncol. 2009; 1:23.
8. Roscoe KJ, Raja S, Tronic B, Dou Y. Single F-18 fluorodeoxyglucose positron emission tomography hypermetabolic focus containing metastatic papillary thyroid cancer within a primary scarring adenocarcinoma lung cancer. Clin Nucl Med. 2006; 31:359–360.
9. Ogawa M, Hori H, Hirayama M, Kobayashi M, Shiraishi T, Watanabe Y, Komada Y. Anaplastic transformation from papillary thyroid carcinoma with increased serum CA19-9. Pediatr Blood Cancer. 2005; 45:64–67.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download