1. DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981; 30:1000–1007.
2. DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985; 76:149–155.
3. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver: a collusion responsible for NIDDM. Diabetes. 1988; 37:667–687.
4. Harita N, Hayashi T, Sato KK, Nakamura Y, Yoneda T, Endo G, Kambe H. Lower serum creatinine is a new risk factor of type 2 diabetes: the Kansai healthcare study. Diabetes Care. 2009; 32:424–426.
5. Solerte SB, Fioravanti M, Locatelli E, Bonacasa R, Zamboni M, Basso C, Mazzoleni A, Mansi V, Geroutis N, Gazzaruso C. Improvement of blood glucose control and insulin sensitivity during a long-term (60 weeks) randomized study with amino acid dietary supplements in elderly subjects with type 2 diabetes mellitus. Am J Cardiol. 2008; 101(11A):82E–88E.
6. Kaplan LA, Pesce AJ, Kazmierczak SC. Clinical chemistry: theory, analysis, correlation. 4th ed. St. Louis: Mosby;2003.
7. American Diabetes Association. Standards of medical care in diabetes: 2010. Diabetes Care. 2010; 33 Suppl 1:S11–S61.
8. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: modification of diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–470.
9. Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010; 5:e10805.
10. Dominguez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J Cardiometab Syndr. 2007; 2:183–189.
11. Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Members of the Florey Adelaide Male Ageing Study. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009; 58:1013–1022.
12. Kim TN, Park MS, Lim KI, Yang SJ, Yoo HJ, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Res Clin Pract. 2011; 93:285–291.
13. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes: findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011; 96:2898–2903.
14. Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond). 2009; 33:885–892.
15. Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care. 2010; 33:1652–1654.
16. Haderslev KV, Haderslev PH, Staun M. Accuracy of body composition measurements by dual energy x-ray absorptiometry in underweight patients with chronic intestinal disease and in lean subjects. Dyn Med. 2005; 4:1.
17. Choi KM. Sarcopenia and sarcopenic obesity. Endocrinol Metab. 2013; 28:86–89.
18. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010; 39:412–423.
19. Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, Heymsfield S, Kopple JD, Kovesdy CP, Kalantar-Zadeh K. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle. 2013; 4:19–29.
20. Schutte JE, Longhurst JC, Gaffney FA, Bastian BC, Blomqvist CG. Total plasma creatinine: an accurate measure of total striated muscle mass. J Appl Physiol. 1981; 51:762–766.
21. Donadio C, Halim AB, Caprio F, Grassi G, Khedr B, Mazzantini M. Single- and multi-frequency bioelectrical impedance analyses to analyse body composition in maintenance haemodialysis patients: comparison with dual-energy X-ray absorptiometry. Physiol Meas. 2008; 29:S517–S524.
22. Keshaviah PR, Nolph KD, Moore HL, Prowant B, Emerson PF, Meyer M, Twardowski ZJ, Khanna R, Ponferrada L, Collins A. Lean body mass estimation by creatinine kinetics. J Am Soc Nephrol. 1994; 4:1475–1485.
23. Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, Nissenson AR, Krishnan M, Kopple JD, Mehrotra R, Anker SD. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010; 85:991–1001.
24. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans RO, Bakker SJ. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009; 207:534–540.

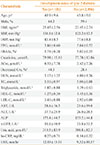
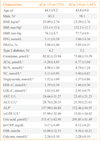





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download