Abstract
A 48-year-old woman was incidentally found to have bilateral adrenal masses, 2.8 cm in diameter on the right, and 2.3 cm and 1.7 cm in diameter on the left, by abdominal computed tomography. The patient had a medical history of hypertension, which was not being controlled by carvedilol, at a dose of 25 mg daily. She presented with signs and symptoms that suggested Cushing Syndrome. We diagnosed adrenocorticotropic hormone (ACTH)-independent Cushing Syndrome based on the results of basal and dynamic hormone tests. Adrenal vein sampling (AVS) was performed to localize a functioning adrenal cortical mass. AVS results were consistent with hypersecretion of cortisol from both adrenal glands, with a cortisol lateralization ratio of 1.1. Upon bilateral laparoscopic adrenalectomy, bilateral ACTH-independent adrenal adenomas were found. The patient's signs and symptoms of Cushing Syndrome improved after surgery just as the blood pressure was normalized. After surgery, the patient was started on glucocorticoid and mineralocorticoid replacement therapy.
Adrenocorticotropic hormone (ACTH)-independent Cushing Syndrome constitutes about 10% to 15% of endogenous Cushing Syndrome cases, and unilateral cortisol-secreting adenomas are the most common cause [1]. ACTH-independent Cushing Syndrome induced by unilateral cortisol-secreting adenomas is relatively easy to diagnose and treat, but when bilateral adrenal masses are found in a patient seemingly with ACTH-independent Cushing Syndrome, it becomes difficult to accurately diagnose and determine the exact location of the functional lesion. In particular, functional and nonfunctional adrenal tumors are difficult to differentiate through radiological examinations such as computed tomography (CT) or magnetic resonance imaging (MRI).
In Korea, Park et al. [2] reported one case of Cushing Syndrome induced by functional black adrenal adenoma with nonfunctional adrenal adenoma on the opposite side, while Won et al. [3] reported bilateral cortisol-secreting adenomas, without any functional assessment.
We diagnosed ACTH-independent Cushing Syndrome in a 48-year-old female patient who had bilateral adrenal masses incidentally identified based on clinical symptoms and endocrinology examination during a hospital visit. We identified the presence of bilateral cortisol-secreting adenomas through blood sampling from the adrenal vein of the patient. The patient was treated by bilateral total adrenalectomy. Here we report on the details of this case, along with a literature review.
The patient had been diagnosed with hypertension 3 years prior, and had been receiving carvedilol 25 mg as prescribed by a private clinic. However, the patient's blood pressure (BP) had not been under control for about 3 months prior to the visit at our hospital. A year prior, the patient's menstrual cycle had become irregular and her weight had increased by 5 kg in the last year. The patient had visited our hospital due to the identification of bilateral adrenal masses from abdominal CT during a routine health examination.
At the time of the visit to our hospital, the patient's height was 150 cm, weight was 53.8 kg, BP was 145/95 mm Hg, pulse rate was 72 beats per minute, respiratory rate was 20 times per minute, and body temperature was 37.1℃. She had an alert mental state, no visible signs for acute or chronic illness and no abnormal findings for skin, sclera and conjunctiva. Examination of the head and neck revealed a moon face, while a buffalo hump deformity was observed upon thoracic examination. There was no specific finding on the abdominal examination and no edema of extremities was identified. The patient had no accompanying muscle weakness or sensory deterioration.
The peripheral blood test performed at the time of visit to the hospital showed the following; white blood cell, 6,450/mm3; hemoglobin, 12.9 g/dL; hematocrit, 41.6%; and platelet, 289,000/mm3. The serum biochemical assay showed the following; calcium, 9.4 mg/dL; phosphorus, 3.3 mg/dL; total protein, 7.0 g/dL; albumin, 4.2 g/dL; aspartate aminotransferase, 21 IU/L; alanine aminotransferase, 19 IU/L; alkaline phosphatase, 43 IU/L; blood urea nitrogen, 10 mg/dL; creatinine, 0.7 mg/dL; sodium, 144 mEq/L; potassium, 3.4 mEq/L; and chlorine, 102 mEq/L.
The hormone test indicated that the morning baseline blood cortisol was 15.7 µg/dL and ACTH was less than 5 pg/mL. The 24-hour urine free cortisol excretion increased to 198.7 µg/day (normal range, 23 to 135). In addition, the following were in the normal range; metanephrine, 63 µg/day (normal range, 52 to 341); normetanephrine, 176 µg/day (normal range, 88 to 444); epinephrine, 3.1 µg/day (normal range, 0 to 20); norepinephrine, 28.9 µg/day (normal range, 15 to 80); and vanillylmandelic acid, 5.4 mg/day (normal range, 0 to 8). The 24-hour urine creatinine was 0.5 g/day (normal range, 0.8 to 1.8) and the creatinine clearance was calculated to be 122.4 mL/min (normal range, 75 to 125), indicating that the urine sampling was done relatively well. The plasma aldosterone concentration was 7.5 ng/dL and plasma renin activity was 0.4 ng/mL/hr, indicating that the aldosterone/renin activity ratio was 18.7 and there was no finding of primary aldosteronism.
The serum cortisol after an overnight 1 mg dexamethasone suppression test was 14.5 µg/dL, which was not suppressed. The low-dose dexamethasone suppression test demonstrated that the 24-hour urine free cortisol excretion increased to 397.8 µg/day and the blood cortisol concentration increased to 21.1 µg/dL, so we diagnosed the patient's condition as Cushing Syndrome. In the high-dose dexamethasone suppression test, the urine free cortisol excretion and blood cortisol concentration were not suppressed (Table 1).
Bilateral adrenal tumors were identified on adrenal CT, with one sized 2.8×2.0 cm on the right side and the others sized 2×2.3 cm and 1.7×1.4 cm, respectively, on the left side (Fig. 1). There were no abnormal findings from the Sella MRI.
Adrenal vein sampling (AVS) was done in order to identify the location of a functional tumor secreting cortisol in between the bilateral adrenal tumors. AVS was conducted without an ACTH stimulus on the day after the high-dose dexamethasone suppression test. The blood cortisol concentration was measured by sampling the blood twice from catheterization to the femoral vein and bilateral adrenal veins (Table 2).
When the bone density was measured by dual energy X-ray absorptiometry, osteopenia was found based on the finding that the T score of lumber spine was -2.1, T score of femoral neck was -1.0 and T score of the total hip was -0.7.
As the adrenal vein cortisol measured from AVS was eight times higher than that of the inferior vena cava, it was determined that the AVS was done selectively. The blood cortisol concentration was measured as high from the bilateral adrenal veins with AVS. Therefore, we diagnosed the patient with Cushing Syndrome induced by bilateral cortisol-secreting adenomas. To treat the patient, we removed all of the bilateral adrenal tumors by laparoscopic bilateral adrenalectomy. Immediately after the surgery, the patient was discharged because there was no specific complication while implementing supplementary therapy with prednisolone and fludrocortisone, and we had discontinued the antihypertensive agent as the BP had improved to 120/80 mm Hg at the 2-month follow-up observation. At 5 months postoperatively, the blood cortisol concentration was 5.0 µg/dL, ACTH was 19.0 pg/mL, the plasma aldosterone concentration was 15.0 ng/dL, and the plasma renin activity was 3.0 ng/mL/hr, indicating no evidence of recurrence. The patient is currently under follow-up study as an outpatient while receiving prednisolone 5 to 2.5 mg and fludrocortisone 0.1 mg.
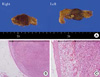
A single mass was found on each of the adrenal glands and their sizes were 2.6×2.3×1.7 cm for the left side mass and 2.6×2.5×1.6 cm for the right side mass. Based on the visual findings, the left mass was in the shape of a dumbbell with a narrowed mid-part, showing a light brown cross-section, and the right mass had a brown cross-section (Fig. 2A).
From the optical microscopic findings, the bilateral adenomas were composed of large round vacuolar cells of consistent shape and size, and with good differentiation, whereas the normal tissues of the adrenal cortex around the adenoma were atrophic. As an atypical nucleus and necrosis, hemorrhage, or reverse differentiation were not found, the findings were appropriate for adrenal adenoma (Fig. 2B).
This case was a patient with bilateral adrenal tumors (diameters of 1.7, 2.3, and 2.8 cm) that were found incidentally on abdominal CT. In addition, the patient had symptoms such as a moon face, irregular menstruation, bone mass reduction and hypertension, and the patient was diagnosed with ACTH-independent Cushing Syndrome through endocrinological examinations. When bilateral adrenal masses are found in a patient presenting with symptoms of ACTH-independent Cushing Syndrome, it is difficult to determine the appropriate treatment. Bilateral adrenal lesions need to be differentiated from ACTH-independent bilateral macronodular adrenal hyperplasia (AIMAH), bilateral primary pigmented nodular adrenocortical hyperplasia (PPNAD), bilateral cortisol-secreting adenomas and adenocarcinoma [4].
PPNAD occurs mainly in patients younger than 30 years old and shows micronodules sized ≤1 cm mostly upon adrenal CT scan [5]. In the case of AIMAH, multiple nodules can be found by adrenal CT scan, and are characterized by the rough contour of the adrenal gland. They cannot be differentiated notably from adrenal adenomas, but they still can be differentiated from adrenal adenomas in that adrenal hyperplasia is found in between nodules from postoperative pathological findings [6].
In the case of bilateral adrenal adenomas, it is necessary to differentiate between bilateral cortisol-secreting adenomas, unilateral cortisol-secreting adenoma, and nonfunctional adenomas, and to identify the lesion to resect. Young et al. [7] had reported that AVS was helpful for differentiation of bilateral cortisol-secreting adenomas from among cases of ACTH-independent Cushing Syndrome with bilateral adrenal masses. When the cortisol ratio of adrenal vein to peripheral vein from the AVS was ≥6.5, it was defined as a cortisol-secreting adenoma. If the ratio was ≤3.3, it was considered a nonfunctional adenoma and in the case of AIMAH, the mean of the aforementioned three cases was 5.1 [7]. In this case, the cortisol ratio of adrenal vein/peripheral vein was 7.8 on the right side and 7.4 on the left side, which is appropriate for bilateral cortisol-secreting adenomas. Young et al. [7] had reported that when there were bilateral adrenal masses, and when the bilateral adrenal vein lateralization ratio was ≤2.0, it was bilateral cortisol hypersecretion. In this case, the bilateral adrenal vein lateralization ratio was about 1.1, which is indicative of bilateral cortisol hypersecretion.
Based on postbilateral total adrenalectomy gross and microscopic histological findings, the patient had typical adrenal adenomas composed of lipid-containing large vacuolar cells with good differentiation, without any evidence of PPNAD or AIMAH and no atypical mitosis, necrosis, hemorrhage, or reverse differentiation. Therefore, it was possible to diagnose this case as Cushing Syndrome induced by bilateral cortisol-secreting adenomas. The patient is currently under regular follow-up observation, and is receiving glucocorticoid and mineralocorticoid supplementation therapy.
To date, in Korea, there has not been any reported case of ACTH-independent Cushing Syndrome with bilateral cortisol-secreting adenomas incidentally discovered through endocrinological examination and AVS. Our experience demonstrates that AVS is a useful test for suitable diagnosis and treatment of ACTH-independent Cushing Syndrome with bilateral adrenal masses.
In summary, we diagnosed ACTH-independent Cushing Syndrome in a 48-year-old female patient who had visited the hospital with incidentally discovered bilateral adrenal masses as the main complaint. We identified the bilateral cortisol-secreting adenomas through AVS and performed bilateral total adrenalectomy to treat the patient. AVS is a useful test for accurate diagnosis and treatment of patients with ACTH-independent Cushing Syndrome accompanied by bilateral adrenal masses.
Figures and Tables
Fig. 1
Adrenal computed tomography (CT). Axial images from noncontrast-enhanced adrenal CT show a right adrenal nodule 2.8 cm in diameter, and two left adrenal nodules, 2.3 and 1.7 cm, respectively, in diameter (arrows).
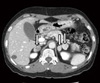
Fig. 2
(A) Gross finding of tumors. The right adrenal mass was well-circumscribed and brownish, measuring 2.6×2.5×1.6 cm in size. A left adrenal mass was dumbbell-shaped and yellowish, measuring 2.6×2.3×1.7 cm in size. (B, C) Microscopic finding of right adrenal adenoma. Microscopically, both masses were predominantly composed of large clear cells with well-differentiated cells. Nuclear atypia, mitoses, necrosis and features of vascular invasion were not found. Double-headed arrow indicates atrophy of normal adrenal tissue due to tumor (B, H&E stain, ×40; C, H&E stain, ×200).
References
1. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing Syndrome. Lancet. 2006. 367:1605–1617.
2. Park DJ, Park KS, Nam KJ, Kim SY, Cho BY, Lee HG, Yoon YK, Oh SK. A case of black adrenocortical adenoma causing Cushing Syndrome with contralateral nonfuncioning adenoma. J Korean Soc Endocrinol. 1999. 14:410–417.
3. Won UH, Kim JA, Rha SY, Chung YS, Lee HC, Huh KB, Park JS, Sung SH. A case of Cushing Syndrome due to bilateral adrenocortical adnomas. Korean J Med. 1994. 47:395–400.
4. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing Syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008. 93:1526–1540.
5. Carney JA, Young WFJ. Primary pigmented nodular adrenocortical disease and its associated conditions. Endocrinologist. 1992. 2:6–21.
6. Melmed S, Williams RH. Williams textbook of endocrinology. 2011. 12th ed. Philadelphia: Elsevier/Saunders;479–544. Chapter 15, The adrenal cortex.
7. Young WF Jr, du Plessis H, Thompson GB, Grant CS, Farley DR, Richards ML, Erickson D, Vella A, Stanson AW, Carney JA, Abboud CF, Carpenter PC. The clinical conundrum of corticotropin-independent autonomous cortisol secretion in patients with bilateral adrenal masses. World J Surg. 2008. 32:856–862.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download