Abstract
The majority of the vitamin D in our body is produced by cutaneous synthesis in response to sunlight. As more and more people live in cities and spend the bulk of their time indoors, it can be difficult to get sufficient sun exposure for adequate cutaneous production of vitamin D. Therefore, vitamin D insufficiency has become a very common health problem worldwide. The Korea National Health and Nutrition Examination Survey IV 2008 showed that the prevalence of vitamin D insufficiency, defined as a serum 25-hydroxyvitamin D [25(OH)D] level below 50 nmol/L, was 47.3% in males and 64.5% in females. Only 13.2% of males and 6.7% of females had a serum 25(OH)D level of greater than 75 nmol/L. In Korea, vitamin D insufficiency was more prevalent in young adults than in elderly people, likely due to the indoor lifestyle of younger people. Compared with the United States and Canada, Korea has a lower mean 25(OH)D level and a higher prevalence of vitamin D insufficiency. To improve the vitamin D status of the Korean population, more aggressive policies on food fortification and vitamin D supplementation are needed.
Vitamin D is well known for its important role in bone and mineral metabolism. It promotes calcium and phosphate absorption in the intestine and maintains an adequate concentration of these minerals in circulation to allow for normal bone mineralization. Accordingly, low vitamin D status is clinically associated with osteoporosis and fractures. Furthermore, vitamin D deficiency can lead to rickets in children and osteomalacia in adults. Recently, many nonskeletal actions of vitamin D on cellular proliferation and differentiation, muscle function, and immunity have also been suggested [1]. Several clinical studies have shown that vitamin D deficiency or insufficiency is associated with an increased risk of nonskeletal health conditions, including cardiovascular diseases, diabetes mellitus, cancers, infections, and autoimmune diseases [2-15].
Although vitamin D can be obtained from dietary sources such as fatty fish and meat, the vitamin D in the human body is mainly acquired by cutaneous synthesis in response to sunlight. Once vitamin D is formed in the skin surface, it undergoes two sequential hydroxylations in the liver and the kidney in order to become biologically active. As people increasingly live in cities and spend the majority of their time indoors, it has become more difficult to get sufficient sun exposure for adequate cutaneous production of vitamin D. Furthermore, even when outdoors, many people use sunscreen and avoid direct exposure to sunlight due to concerns about skin cancer and aging. Therefore, there is a very high prevalence of vitamin D insufficiency worldwide. In particular, as Korea has experienced rapid industrialization and economic development over the past few decades, vitamin D insufficiency has become a public health problem not only in elderly people, but also in younger generations due to changes in lifestyle and occupations [16].
It has been well established that vitamin D status is best assessed using the serum concentration of 25-hydroxyvitamin D [25(OH)D], which is produced in the liver and reflects vitamin D obtained from both cutaneous synthesis and dietary sources [17]. Although previous studies have investigated what constitutes a sufficient level of 25(OH)D using several criteria, including serum parathyroid hormone suppression, calcium absorption, bone mineral density, and fall or fracture rates [18,19], there is no clear consensus on what level of serum 25(OH)D is sufficient. According to the World Health Organization, serum 25(OH)D levels below 25 nmol/L are deficient and levels below 50 nmol/L are insufficient. However, there have been many proposals to increase the lower threshold of sufficient 25(OH)D level to upwards of 75 nmol/L [20]. Bischoff-Ferrari et al. [19] suggested that the most advantageous serum 25(OH)D levels begin at 75 nmol/L, with the optimal level ranging from between 90 and 100 nmol/L. Most recently, the Institute of Medicine in the United States suggested that a serum 25(OH)D level of 50 nmol/L is sufficient to ensure bone health after an extensive literature review of vitamin D [21]. Therefore, it is still controversial whether sufficient vitamin D status should be defined as a serum 25(OH)D level of above 50 or 75 nmol/L.
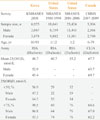
Vitamin D status in the Korean population has been previously investigated based on The Korea National Health and Nutrition Examination Surveys IV conducted in 2008 (Table 1) [16]. The mean serum 25(OH)D level measured by radioimmunoassay (RIA) (DiaSorin, Stillwater, MN, USA) was 52.9 nmol/L in males and 45.4 nmol/L in females. The prevalence of vitamin D insufficiency, defined as a serum 25(OH)D level below 50 nmol/L, was 47.3% in males and 64.5% in females. With a serum 25(OH)D level of 75 nmol/L as the threshold, the prevalence of vitamin D insufficiency rose to 86.8% in men and 93.3% in women. Interestingly, the study showed that serum 25(OH)D level increases with age from 20-29 to 60-69 years in both sexes. Furthermore, contrary to the common belief that vitamin D insufficiency is more prevalent in elderly people, vitamin D insufficiency was most prevalent in the 20 to 29 age group (65.0% in males and 79.9% in females, with 50 nmol/L as the threshold of vitamin D insufficiency). In the same study, vitamin D insufficiency was more prevalent among those who work indoor occupations such as sales, service, administration, clerical work, or specialists compared with those who have outdoor occupations such as agriculture, forestry, or fishing.
Although vitamin D status has been studied in many countries worldwide, relatively few studies have assessed the vitamin D status of a population based on large representative samples. In the United States and Canada, the vitamin D status of the population has been investigated in nationwide surveys (Table 1) [22,23].
In the United States, vitamin D status was investigated in the National Health and Nutrition Examination Survey (NHANES) (Table 1). Serum 25(OH)D levels were measured using RIA (DiaSorin) in the NHANES 1988 to 1994 and the NHANES 2001 to 2006 [22]. The mean serum 25(OH)D level was 60.7 nmol/L in 1988 to 1994 and 55.2 nmol/L in 2001 to 2006. The prevalence of vitamin D insufficiency, defined as a serum 25(OH)D level of less than 50 nmol/L, was 29% in the NHANES 1988 to 1994 and 32% in the NHANES 2001 to 2006. With a serum 25(OH)D level of 75 nmol/L as the threshold, the prevalence of vitamin D insufficiency was 69% in the NHANES 1988 to 1994 and 76% in the NHANES 2001 to 2006. Overall, there was a trend toward an increased prevalence of vitamin D insufficiency in 2001 to 2006 compared to 1988 to 1994.
In Canada, vitamin D status was investigated in the Canadian Health Measures Survey from 2007 to 2009 (Table 1). Serum 25(OH)D levels were measured by a chemiluminescence assay using the LIAISON 25-Hydroxyvitamin D TOTAL assay (DiaSorin) [23]. The mean serum 25(OH)D level among Canadians was 67.7 nmol/L. For both sexes, the serum level of 25(OH)D was highest among children and seniors, and lowest in those aged 20 to 39 years. The prevalence of serum 25(OH)D level below 75 nmol/L was 67.0% in males and 62.2% in females and highest in those aged 20 to 39 years. Meanwhile, comparison between the RIA and LIAISON methods showed an average bias of 4.8±16 nmol/L with the LIAISON method giving higher values [24].
An individual's vitamin D status is determined by several factors related to cutaneous synthesis or dietary intake of vitamin D. Accordingly, traditional risk factors for poor vitamin D status include limited sun exposure, higher latitude, winter season, darker skin pigmentation, sunscreen use, skin-covering clothing, and a diet low in fish and dairy products [25,26]. Dietary intake is more important when there is less sunshine exposure. Air pollution probably also plays a role in large cities [27]. Older age is commonly suggested to be a risk factor for vitamin D insufficiency because the cutaneous synthesis of vitamin D declines with age [1,26,28]. Aging can decrease the capacity of the skin to produce previtamin D in response to ultraviolet radiation by greater than 2-fold [28]. However, in Korea and Canada, vitamin D insufficiency was more prevalent among young adults than in the older people. Although the cause of this finding is not clear, it may be due to behavioral factors such as an indoor lifestyle, sunscreen use, outdoor activity, or dietary habits. Previously, the National Diet and Nutrition Survey 1992 to 2001 of the United Kingdom also showed that vitamin D insufficiency was most prevalent among young adults aged 19 to 24 years as well as in elderly people over 85 years of age [25].
Despite growing awareness of the multiple health benefits of vitamin D, vitamin D insufficiency remains a major health problem worldwide. It is not only a health concern of elderly people, but has been shown to affect all age groups. In Korea, vitamin D insufficiency is an especially common health condition. Compared with the United States and Canada, Korea has a lower mean 25(OH)D level and a higher prevalence of vitamin D insufficiency, although it is important to exercise caution when comparing results from surveys conducted in different laboratories or using different methods (Table 1). Therefore, it should be a critical public health issue to improve the vitamin D status of the Korean population. Due to the heavily indoor lifestyle of modern society, it is difficult to get adequate sunlight exposure for sufficient vitamin D production. Furthermore, it is also challenging to change the dietary habits of a population in order to increase the intake of foods rich in vitamin D such as fish. More realistic ways of improving vitamin D status include food fortification and supplementation with vitamin D. An aggressive national policy on food fortification for the general population should be adopted. Likewise, the promotion of vitamin D supplementation is necessary, especially for specific groups at risk for vitamin D insufficiency.
References
1. Holick MF. Vitamin D deficiency. N Engl J Med. 2007. 357:266–281.
2. Holick MF. Vitamin D: a D-lightful vitamin for health. Endocrinol Metab. 2012. 27:255–267.
3. Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, Warburton EA. Reduced vitamin D in acute stroke. Stroke. 2006. 37:243–245.
4. Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007. 49:1063–1069.
5. Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008. 168:1174–1180.
6. Mattila C, Knekt P, Mannisto S, Rissanen H, Laaksonen MA, Montonen J, Reunanen A. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007. 30:2569–2570.
7. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007. 92:2017–2029.
8. Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. 2000. 11:847–852.
9. Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006. 98:451–459.
10. Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007. 167:1050–1059.
11. Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, Rinaldi S, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Kaaks R, Linseisen J, Boeing H, Bergmann MM, Trichopoulou A, Misirli G, Trichopoulos D, Berrino F, Vineis P, Panico S, Palli D, Tumino R, Ros MM, van Gils CH, Peeters PH, Brustad M, Lund E, Tormo MJ, Ardanaz E, Rodriguez L, Sanchez MJ, Dorronsoro M, Gonzalez CA, Hallmans G, Palmqvist R, Roddam A, Key TJ, Khaw KT, Autier P, Hainaut P, Riboli E. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ. 2010. 340:b5500.
12. Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000. 355:618–621.
13. Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamaki H, Ylikomi T. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007. 86:714–717.
14. Ginde AA, Mansbach JM, Camargo CA Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009. 169:384–390.
15. Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol. 2008. 20:532–537.
16. Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, Kim KJ, Rhee Y, Lim SK. Vitamin D insufficiency in Korea: a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011. 96:643–651.
17. Iqbal SJ. Vitamin D metabolism and the clinical aspects of measuring metabolites. Ann Clin Biochem. 1994. 31(Pt 2):109–124.
18. Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005. 16:713–716.
19. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006. 84:18–28.
20. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004. 80:6 Suppl. 1678S–1688S.
21. Ross AC. Institute of Medicine (U.S.). Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. 2011. Washington DC: National Academies Press.
22. Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012. 142:498–507.
23. Langlois K, Greene-Finestone L, Little J, Hidiroglou N, Whiting S. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2010. 21:47–55.
24. Sarafin K, Hidiroglou N, Brooks SPJ. A Comparison of Two Immunoassays for Analysing Plasma 25-hydroxyvitamin D. Open Clin Chem J. 2011. 4:45–49.
25. Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008. 66:10 Suppl 2. S153–S164.
26. Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J. IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009. 20:1807–1820.
27. Hosseinpanah F, Pour SH, Heibatollahi M, Moghbel N, Asefzade S, Azizi F. The effects of air pollution on vitamin D status in healthy women: a cross sectional study. BMC Public Health. 2010. 10:519.
28. MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985. 76:1536–1538.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download