Abstract
Background
Follicular thyroid tumors harbor several genetic alterations such as RAS mutations and PAX8/PPARγ rearrangement. The aims of our study were to investigate the prevalence of RAS mutations and PAX8/PPARγ rearrangement in follicular thyroid tumors and to correlate RAS mutations and/or PAX8/PPARγ rearrangement with clinicopathologic features in Korean patients with follicular thyroid carcinomas.
Methods
RAS mutations were investigated by polymerase chain reaction and DNA sequencing in surgical specimens of 37 follicular thyroid carcinomas (FTCs) and 16 follicular thyroid adenomas (FTAs). PAX8/PPARγ rearrangement was analyzed by fluorescent in situ hybridization in surgical specimens of 31 FTCs and 13 FTAs.
Results
RAS mutations were detected in 30% (11 of 37) of FTCs and 19% (three of 16) of FTAs. Three of 11 FTC patients with RAS mutations died of thyroid cancer, but none of the 26 FTC patients without RAS mutations. PAX8/PPARγ rearrangement was found in 10% (three of 31) of FTCs, but in none of the 13 FTAs. All three FTC patients with PAX8/PPARγ rearrangement remained in complete remission during follow-up. There were no FTC patients with both RAS mutations and PAX8/PPARγ rearrangement.
Conclusion
The prevalence of RAS mutations in our series of follicular tumors was similar to previous studies. The frequency of PAX8/PPARγ rearrangements in our group of FTC was lower than previous western reports, but higher than Japanese reports. RAS mutations may be associated with hematogeneous metastasis and poor survival while PAX8/PPARγ rearrangement may be related to more favorable prognosis in Korean patients with FTCs.
Figures and Tables
Fig. 1
Sequence chromato-gram of NRAS exon-2 encompassing codon 61 shows a heterozygosity composed of an altered nucleotide 'A' and a wild-type nucleotide 'C', resulting in Q61K mutation.

Fig. 2
Interphase fluorescence in situ hybridization (FISH) analysis demonstrating the absence of a t(2;3)(q13;p25) translocation and its presence in a follicular thyroid carcinoma (FTC). The locations of the BAC probes used for the FISH fusion assays are shown in relation to PAX8 and PPARγ next to the ideograms of normal chromosomes 2 and 3. A 2q13 probe (83_K08), centromeric of PAX8, was labeled with fluorescein-12-dUTP (green), and a 3p25 probe (26_O22), telomeric of PPARγ, was labled with Texas Red-5-dUTP (red). Nuclei from a follicular thyroid adenoma in which t(2;3)(q13;p25) is absent are shown in A, whereas B demonstrates nuclei form an FTC in which t(2;3)(q13;p25) is present (arrow), as demonstrated by the adjacently located green (83_K08) and red (26_O22) hybridization signals.
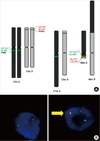
Fig. 3
Clinical outcomes at last follow-up between RAS point mutations (+) and RAS point mutation (-) in patients with follicular thyroid carcinomas.

Fig. 4
PAX8/PPARγ rearrangement with or without other aberrant signal patterns revealed by fluorescence in situ hybridization (F, fusion signal; G, green signal; O, orange signal). A. PAX8/PPARγ rearrangement with the 1F1G1O signal. B. PAX8/PPARγ rearrangement with the 1F1G1O signal. C. PAX8/PPARγ rearrangement with the 2F2G1O signal pattern. D. PAX8/PPARγ rearrangement along with different numerical gains (2F1G1O [left], 2F2G1O [middle], and 2F1G1O [right]). E. PAX8/PPARγ rearrangement with the gains of signals, showing 3G3O (upper two cells), and 3G4O (lower two cells) signal patterns. F. PAX8/PPARγ rearrangement with the gains of signals showing multiple O signals (white arrow), indicating the amplification of the PPARγ.
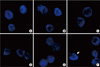
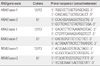
Table 3
Comparison of clinicopathologic features of 37 FTC patients according to RAS point mutations

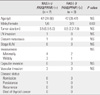
Table 7
Prevalence of RAS mutations and PAX8/PPARγ rearrangement

FTA, follicular thyroid adenoma; FTC, follicular thyroid carcinoma; PAX8/PPARγ (+), positive for PAX8/PPARγ rearrangement; RAS (+), positive for RAS mutations; RAS (+)/PAX8/PPARγ (+), positive for both RAS mutations and PAX8/PPARγ rearrangement; RAS (-)/PAX8/PPARγ (-), negative for both RAS mutations and PAX8/PPARγ rearrangement.
References
1. Hong EK, Lee JD. A national study on biopsy-confirmed thyroid diseases among Koreans: an analysis of 7758 cases. J Korean Med Sci. 1990. 5:1–12.
2. Choi CW, Moon DH, Lee MC, Cho BY, Koh CS, Lee MH, Oh SK, Choi KJ, Park SH, Kim YI. Clinical study on thyroid cancer (the 3rd report). Korean J Nucl Med. 1986. 20:59–65.
3. Vini L, Harmer C. Management of thyroid cancer. Lancet Oncol. 2002. 3:407–414.
4. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993. 328:553–559.
5. Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008. 21:Suppl 2. S37–S43.
6. Barbacid M. Ras genes. Annu Rev Biochem. 1987. 56:779–827.
7. Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, Fletcher JA. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science. 2000. 289:1357–1360.
8. Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW 2nd, Tallini G, Kroll TG, Nikiforov YE. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003. 88:2318–2326.
9. Marques AR, Espadinha C, Catarino AL, Moniz S, Pereira T, Sobrinho LG, Leite V. Expression of PAX8-PPAR gamma 1 rearrangements in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab. 2002. 87:3947–3952.
10. Dwight T, Thoppe SR, Foukakis T, Lui WO, Wallin G, Höög A, Frisk T, Larsson C, Zedenius J. Involvement of the PAX8/peroxisome proliferatoractivated receptor gamma rearrangement in follicular thyroid tumors. J Clin Endocrinol Metab. 2003. 88:4440–4445.
11. Park KY, Koh JM, Kim YI, Park HJ, Gong G, Hong SJ, Ahn IM. Prevalences of Gs alpha, ras, p53 mutations and ret/PTC rearrangement in differentiated thyroid tumours in a Korean population. Clin Endocrinol (Oxf). 1998. 49:317–323.
12. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009. 19:1167–1214.
13. DeLellis RA, Lloyd RV, Heinz PU. WHO classification of tumours, pathology and genetics of tumours of endocrine organs. 2004. Lyon: IARC Press;73–76.
14. Lemoine NR, Mayall ES, Wyllie FS, Farr CJ, Hughes D, Padua RA, Thurston V, Williams ED, Wynford-Thomas D. Activated ras oncogenes in human thyroid cancers. Cancer Res. 1988. 48:4459–4463.
15. Suarez HG, du Villard JA, Severino M, Caillou B, Schlumberger M, Tubiana M, Parmentier C, Monier R. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene. 1990. 5:565–570.
16. Esapa CT, Johnson SJ, Kendall-Taylor P, Lennard TW, Harris PE. Prevalence of Ras mutations in thyroid neoplasia. Clin Endocrinol (Oxf). 1999. 50:529–535.
17. Manenti G, Pilotti S, Re FC, Della Porta G, Pierotti MA. Selective activation of ras oncogenes in follicular and undifferentiated thyroid carcinomas. Eur J Cancer. 1994. 30A:987–993.
18. Namba H, Rubin SA, Fagin JA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol. 1990. 4:1474–1479.
19. Shi YF, Zou MJ, Schmidt H, Juhasz F, Stensky V, Robb D, Farid NR. High rates of ras codon 61 mutation in thyroid tumors in an iodide-deficient area. Cancer Res. 1991. 51:2690–2693.
20. Said S, Schlumberger M, Suarez HG. Oncogenes and anti-oncogenes in human epithelial thyroid tumors. J Endocrinol Invest. 1994. 17:371–379.
21. Vasko V, Ferrand M, Di Cristofaro J, Carayon P, Henry JF, de Micco C. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J Clin Endocrinol Metab. 2003. 88:2745–2752.
22. Garcia-Rostan G, Zhao H, Camp RL, Pollan M, Herrero A, Pardo J, Wu R, Carcangiu ML, Costa J, Tallini G. Ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003. 21:3226–3235.
23. Karga H, Lee JK, Vickery AL Jr, Thor A, Gaz RD, Jameson JL. Ras oncogene mutations in benign and malignant thyroid neoplasms. J Clin Endocrinol Metab. 1991. 73:832–836.
24. Saavedra HI, Knauf JA, Shirokawa JM, Wang J, Ouyang B, Elisei R, Stambrook PJ, Fagin JA. The RAS oncogene induces genomic instability in thyroid PCCL3 cells via the MAPK pathway. Oncogene. 2000. 19:3948–3954.
25. Fagin JA. Minireview: branded from the start-distinct oncogenic initiating events may determine tumor fate in the thyroid. Mol Endocrinol. 2002. 16:903–911.
26. Nikiforov YE. Molecular diagnostics of thyroid tumors. Arch Pathol Lab Med. 2011. 135:569–577.
27. French CA, Alexander EK, Cibas ES, Nose V, Laguette J, Faquin W, Garber J, Moore F Jr, Fletcher JA, Larsen PR, Kroll TG. Genetic and biological subgroups of low-stage follicular thyroid cancer. Am J Pathol. 2003. 162:1053–1060.
28. Hibi Y, Nagaya T, Kambe F, Imai T, Funahashi H, Nakao A, Seo H. Is thyroid follicular cancer in Japanese caused by a specific t(2; 3)(q13; p25) translocation generating Pax8-PPAR gamma fusion mRNA? Endocr J. 2004. 51:361–366.
29. Nikiforova MN, Biddinger PW, Caudill CM, Kroll TG, Nikiforov YE. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol. 2002. 26:1016–1023.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download