Abstract
Bisphosphonates are the mainstay of osteoporosis treatment. Despite the fact that bisphosphonates have a relatively good safety record and are tolerated well by the majority of patients, serious adverse events have been associated with their use. A 41-year-old man had been diagnosed with osteoporosis and had taken etidronate 200 mg/day daily for 2 years due to the judgmental error. He was referred for the management of refractory bone pain and generalized muscle ache. Serum calcium, phosphate, 25-hydroxy-vitamin D (25(OH)D), and immunoreactive parathyroid hormone (iPTH) were within normal range. Plain X-ray showed multiple fractures. Whole body bone scan confirmed multiple sites of increased bone uptakes. Tetracycline-labeled bone biopsy showed typical findings of osteomalacia. He was diagnosed with iatrogenic, etidronate-induced osteomalacia. The patient received daily parathyroid hormone (PTH) injection for 18 months. PTH effectively reverses impaired bone mineralization caused by etidronate misuse. Currently, he is doing well without bone pain. Bone mineral density significantly increased, and the increased bone uptake was almost normalized after 18 months. This case seems to suggest that human PTH (1-34) therapy, possibly in association with calcium and vitamin D, is associated with important clinical improvements in patients with impaired bone mineralization due to the side effect of bisphosphonate.
Bisphosphonates are the mainstay of osteoporosis treatment. The efficacy of bisphosphonates has been well demonstrated [1-3]. Though bisphosphonates have a relatively good safety record and are tolerated well by the majority of patients, serious adverse events have been associated with their use. Hence, some unadvertised aspects of bisphosphonates, including their long half-life and their effects on bone physiology need to be addressed. Furthermore, clinicians need to know how to treat a patient who has experienced side effect of this drug. Recently, many studies have emphasized a long-term side effect of bisphosphonate [4-6]. It has been reported that etidronate, one of the first generation of bisphosphonate medication rarely used now, may lead impairment of bone mineralization and subsequent drug-induced osteomalacia [6-8]. We report a case of a patient who developed osteomalacia because of etidronate misuse and was treated effectively by using parathyroid hormone (PTH), an anabolic agent.
A 41-year-old man with a history of cerebral palsy presented with a 1-year history of proximal muscle weakness and generalized bone pain. The symptom of general pain began 1 year ago, especially at the hip and the leg. The pain progressively worsened, and for the past 3 months, the pain interfered with the patient's sleep. The patient had no history of alcohol, smoking, or hypertension. The patient was previously diagnosed with cerebral palsy, and used wheelchair to move. The patient was diagnosed with osteoporosis at another hospital 3 years ago and since then took etidronate disodium (Dinol, Chodang Pharmaceuticals, Seoul, Korea) 200 mg/day daily for 2 years without interval. The patient discontinued the medication 1 year ago. Whole-body bone scan (WBBS) and magnetic resonance imaging (MRI) were performed in another hospital. WBBS showed increased bone remodeling, and MRI of the lower extremities suggested osteoid osteoma at the right distal fibula. However, bone biopsy revealed no malignancy. There was no history of use of steroid, oriental medicine, or other health products. Family history was negative for specific disease.
A review of system of the patient upon the visit revealed general fatigue, as well as pain at the hip and both lower extremities. There was tenderness at cervical spine, lumbar spine, bilateral tibia, and bilateral fibula. Chemistry showed increased alkaline phosphatase (487 IU/L), but was otherwise normal (calcium, 9.1 mg/dL; phosphorus, 4.2 mg/dL; BUN/creatinine, 16.3/0.95 mg/dL; uric acid, 4.7 mg/dL; protein/albumin, 6.9/4.7 mg/dL; Na/K/Cl/tCO2, 143/4.2/107/25 mmol/L). The results of blood tests were as follows: PTH 15.48 pg/mL (10-57), PTH-related peptide < 1.1, 25-hydroxy-vitamin D (25(OH)D) 21.7 ng/mL (9-37.6), osteocalcin 19.54 ng/mL (5.8-33.8). Serum C-terminal telopeptide of collagen type I (CTx) was increased to 2.080 ng/mL (> 0.584). All serum tumor markers were within normal range. The 24-hour urine test revealed a normal tubular reabsorption of phosphorus (TRP = phosphate clearance/creatinine clearance) of 95%.
The dual energy X-ray absorptiometry (DEXA) suggested osteopenia, with T-score of lumbar spine and femur -2.0 and -1.6, respectively. WBBS showed multiple sites with increased uptake, including bilateral rib, cervical spine, lumbar spine, bilateral femur, right fibula, and right sacrum (Fig. 1).
Including the above findings, there were no evidence of malignancy and other causes of osteomalacia, and the patient was suspected with metabolic bone disease; therefore, tetracycline double labeling was planned, but since the patient could not wait 3 weeks due to severe pain, single labeling bone biopsy was performed at the iliac crest in September 16th, 2009. The findings of bone biopsy were consistent with osteomalacia (Fig. 2). After combining the above findings, the patient was ultimately diagnosed with osteomalacia due to etidronate misuse. We first prescribed (Cal-D-vita, Bayer Korea, Seoul, Korea) (calcium carbonate 1,500 mg and cholecalciferol 400 IU) to supplement calcium and vitamin D; however, it was replaced by Calico (Hanmi Pharmaceuticals, Seoul, Korea) (calcitriol 1 µg) for its strong potency and quick response.
In addition, 20 µg of Forsteo (Eli Lilly and Company, Indianapolis, IN, USA) (teriperatide-hPTH [1-34]) was administered subcutaneously to normalize bone mineralization. After 6 months of use, the patient stated that muscle weakness and bone pain has markedly improved. A WBBS performed in March 2010 showed significant decrease in multiple bone remodeling, and both serum CTx and osteocalcin were elevated (Fig. 3). In February 2011, after 18 months of Forsteo use, DEXA showed significant increase in bone mineral density (T-score of lumbar spine from -2.0 to 0.5, femur from -1.6 to -0.4), and WBBS showed multiple abnormal uptake was almost normalized. He is currently followed up as an outpatient without any bone pain.
Etidronate, analogues of inorganic pyrophosphate and a natural inhibitor of bone mineralization, strongly binds to hydroxyapatite, a component of bone mineral, thus inhibiting resorption and potentially affecting mineralization as well [9]. Due to its lesser potency of osteoclast killing, very high dose of etidronate is required for treatment of osteoporosis. The recommended dose is 400 mg/day for 2 weeks every 3 months [1]. However, continuous oral treatment with high doses of etidronate may lead to impairment of bone mineralization (osteomalacia) and the cessation of bone remodeling. Hence, etidronate treatment needs a medication-free-period to prevent impaired mineralization.
In our patient, it is likely that the abnormal bone mineralization was caused by etidronate misuse. This patient had kept taking etidronate 200 mg/day for 2 years without interval (total dose of eitdronate was 146,000 mg). The serum calcium, phosphorus, 25(OH) D and PTH level were within normal range. Furthermore, there was no evidence of any malignancy, hyperparathyroidism, renal phosphorus wasting, renal disease such as renal tubular acidosis or chronic kidney disease, or vitamin D deficiency. Baseline alkaline phosphatase level was elevated but decreased after calcium and vitamin D replacement. Based on a combination of clinical features, laboratory results, radiological findings, and bone histomorphometry [10], we could diagnose him as osteomalacia.
Mineralization defect caused by bisphosphonate could be reversed after drug cessation; however, as etidronate is a first-generation bisphosphonate, it may remain in the bone for several years, or even permanently [11]. The patient still suffered from bone pain with multiple rib fractures even 1 year after cessation of etidronate despite adequate serum level of vitamin D. Therefore, treatment options in addition to spontaneous recovery with vitamin D and calcium replacement should be considered.
In this patient, bone turnover was extremely suppressed and the function of osteoblast, as well as osteoclast, was severely impaired. We considered intermittent PTH treatment [12] as the next approach because many previous report already showed that it could reverse bisphosphonate-related low bone turnover and some disease with impairment of mineralization [6,13,14]. Recently, Jamal and Hodsman [15] also suggested that anabolic agents would be a more plausible approach to the management of adynamic bone disease, an example of very low bone turnover state. Furthermore, there was one case report which improved skeletal mineralization effectively by hPTH (1-34) in adult hypophosphatasia characterized by severe impairment of mineralization [6]. Meanwhile, we believe that hPTH (1-34) was not the only responsible agent for reversing the impaired mineralization of bone in this patient, even though it played the main role. Vitamin D supplement also has some limited beneficial effects on differentiation of osteoblast and remineralization [10].
This case indicates that bone that has been treated with bisphosphonate is still able to show an anabolic response to intermittent PTH and normalize mineralization. Thus, fortunately, the profound bisphosphonate suppression of bone mineralization is at least partly reversible via using PTH.
Figures and Tables
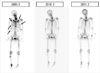 | Fig. 1Whole body bone scan. Administer intermittent human parathyroid hormone (1-34) for 6 and 18 months, increased uptake of multiple bone site was completely resolved. |
References
1. Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, Licata AA, Ross P, Woodson GC 3rd, Yanover MJ, Mysiw WJ, Kohse L, Rao B, Steiger P, Richmond B, Chesnut CH 3rd. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med. 1990. 323:73–79.
2. Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M. The Alendronate Phase III Osteoporosis Treatment Study Group. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995. 333:1437–1443.
3. Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998. 280:2077–2082.
4. Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation. N Engl J Med. 2007. 356:1895–1896.
5. Hong JW, Nam W, Cha IH, Chung SW, Choi HS, Kim KM, Kim KJ, Rhee Y, Lim SK. Oral bisphosphonate-related osteonecrosis of the jaw: the first report in Asia. Osteoporos Int. 2010. 21:847–853.
6. Whyte MP, Mumm S, Deal C. Adult hypophosphatasia treated with teriparatide. J Clin Endocrinol Metab. 2007. 92:1203–1208.
7. Thomas T, Lafage MH, Alexandre C. Atypical osteomalacia after 2 year etidronate intermittent cyclic administration in osteoporosis. J Rheumatol. 1995. 22:2183–2185.
8. Cortet B, Béra-Louville A, Gauthier P, Gauthier A, Marchandise X, Delcambre B. Comparative efficacy and safety study of etidronate and alendronate in postmenopausal osteoporosis. effect of adding hormone replacement therapy. Joint Bone Spine. 2001. 68:410–415.
9. Hiroi-Furuya E, Kameda T, Hiura K, Mano H, Miyazawa K, Nakamaru Y, Watanabe-Mano M, Okuda N, Shimada J, Yamamoto Y, Hakeda Y, Kumegawa M. Etidronate (EHDP) inhibits osteoclastic-bone resorption, promotes apoptosis and disrupts actin rings in isolate-mature osteoclasts. Calcif Tissue Int. 1999. 64:219–223.
10. Ralston SH, Boyce BF, Cowan RA, Fogelman I, Smith ML, Jenkins A, Boyle IT. The effect of 1 alpha-hydroxyvitamin D3 on the mineralization defect in disodium etidronate-treated Paget's disease: a double-blind randomized clinical study. J Bone Miner Res. 1987. 2:5–12.
11. Schenk RK, Olah AJ. What is osteomalacia? Adv Exp Med Biol. 1980. 128:549–562.
12. Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetić K, Müller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001. 16:1846–1853.
13. Gabet Y, Kohavi D, Müller R, Chorev M, Bab I. Intermittently administered parathyroid hormone 1-34 reverses bone loss and structural impairment in orchiectomized adult rats. Osteoporos Int. 2005. 16:1436–1443.
14. Miller PD, Bilezikian JP, Deal C, Harris ST, Ci RP. Clinical use of teriparatide in the real world: initial insights. Endocr Pract. 2004. 10:139–148.
15. Jamal SA, Hodsman AB. Reducing the risk of re-fracture in the dialysis population: is it time to consider therapy with PTH analogues? Semin Dial. 2011. 24:12–15.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download