Abstract
Until the 1980s, polycystic ovary syndrome (PCOS) was considered to be a poorly defined reproductive disorder. During that decade, it was recognized that PCOS was associated with profound insulin resistance and a substantially increased risk for type 2 diabetes mellitus in young women. Accordingly, the mechanisms linking the reproductive and metabolic features of the syndrome became the subject of intense investigation. Insulin is now recognized as a reproductive as well as a metabolic hormone and insulin signaling in the central nervous system participates in normal reproductive function. These insights have been directly translated into a novel therapy for PCOS with insulin sensitizing drugs. Androgens also have reversible metabolic actions to decrease insulin sensitivity and increase visceral fat. Prenatal androgen administration to non-human primates, sheep and rodents produces reproductive and metabolic features of PCOS suggesting that the disorder also has developmental origins. PCOS is highly heritable and male as well as female relatives have reproductive and metabolic phenotypes. A number of confirmed genetic susceptibility loci have now been mapped for PCOS and genes in well-known as well as novel biologic pathways have been implicated in disease pathogenesis.
The association metabolic disturbances and androgen excess in women has been recognized since at least the 18th century [1,2]. In the 1940s, Vague [3] noted that women with male-pattern or android body fat distribution were at increased risk for diabetes and cardiovascular disease (CVD). Rare syndromes of insulin resistant diabetes, hyperandrogenism and acanthosis nigricans were formally described in 1976 [4]. Evaluation of the cellular and molecular defects in insulin action in these syndromes resulted in the identification of mutations in the insulin receptor gene altering its number, binding or function [5,6]. In a subset of these disorders also associated with partial or total lipoatrophy, several Mendelian disorders resulting from mutations in genes regulating adipocyte differentiation or architecture were identified [6,7]. The common feature of these molecularly diverse disorders was hyperinsulinemia implicating insulin in the pathogenesis of the reproductive disturbances [8]. The recognition that women with polycystic ovary syndrome (PCOS) had basal and glucose-stimulated hyperinsulinemia, independent of obesity [9], and acanthosis nigricans [10,11] suggested insulin might also be important in the pathogenesis of this common syndrome of unknown etiology [8].
PCOS is one of the most prevalent endocrine disorders of reproductive age women, affecting approximately 7% of this population [12-14] or to 4 million women ages 15-44 years in the US alone using 2010 Census population estimates. Worldwide PCOS prevalence rates are similar, except in Latinas where they may be even higher [8]. PCOS is diagnosed by its reproductive phenotype of hyperandrogenism, chronic anovulation, and polycystic ovaries (PCO) [8]. It is frequently associated with substantial insulin resistance, pancreatic β-cell dysfunction and obesity [8,15,16]. PCOS is a leading risk factor for metabolic syndrome (MetS) [17-20], a constellation of CVD risk factors associated with visceral adiposity and insulin resistance [21], and type 2 diabetes mellitus (T2D) in adolescent [18, 22] as well as in adult women [23-25]. Women with PCOS appear to be at risk for a number of other conditions associated with insulin resistance, including gestational diabetes, preeclampsia, sleep apnea and non-alcoholic fatty liver disease [26]. Women with epilepsy and bipolar disorder have an increased prevalence of PCOS, independent of medications [27,28].
The metabolic sequelae of PCOS persist after menopause, particularly the increased T2D risk [29-31]. Moreover, several studies of postmenopausal women with features of PCOS suggest that they are indeed at increased risk for CVD [32,33], as would be expected from the increased prevalence of CVD risk factors including obesity, dysglycemia, MetS, T2D, elevated low-density lipoprotein (LDL) levels, and endothelial dysfunction in PCOS [34]. PCOS is highly heritable [35,36] and male as well as female relatives, including infants and children, have metabolic and reproductive phenotypes [35,37-40].
Despite more than 75 years of investigative effort, the etiology(s) of PCOS remain unknown [8]. However, major advances in understanding PCOS have been made in the last 25 years through investigation of the links between its reproductive and metabolic abnormalities. Most importantly, the insight that insulin resistance and the resultant hyperinsulinemia contribute to the reproductive phenotype of PCOS has led to a major new therapeutic modality with insulin-sensitizing agents [15]. However, insulin resistance does not account entirely for the reproductive features of the syndrome. The role of androgens in the pathogenesis of PCOS has been reexamined in recent years since prenatal androgen exposure can create an almost complete phenocopy of PCOS in non-human primates [41]. The well-known familial aggregation of PCOS as well as twin studies indicate that there is also a genetic susceptibility to the disorder [35-37]
The biochemical reproductive phenotype in PCOS is characterized by increased luteinizing hormone (LH) relative to follicle-stimulating hormone (FSH) secretion and hyperandrogenism [42]. There is increased frequency of gonadotropin-releasing hormone (GnRH) secretion that selectively increases LH release, while simultaneously suppressing FSH secretion (Fig. 1) [42]. Pituitary sensitivity to GnRH is also increased, which appears to be estrogen-mediated [42]. Increased LH levels stimulate increased ovarian theca cell androgen production [43]. PCOS theca cells secrete increased androgens, both basally and in response to LH [44].
Acyclic FSH secretion results in arrested ovarian follicular development so that granulosa cells do not develop sufficient aromatase capacity to completely aromatize androgens into estrogens [43]. Often, there is increased adrenal androgen production (Fig. 1) [45]. Androgens play an essential role in the pathogenesis of the increased LH release by causing insensitivity to the normal feedback effects of estrogen and progesterone feedback to slow GnRH frequency in PCOS (Fig. 1); normal feedback can be restored by androgen receptor blockade [46]. Hyperinsulinemia secondary to insulin resistance plays a pivotal role in amplifying both the steroidogenic as well as the gonadotropin secretory abnormalities [8].
There are intrinsic abnormalities in PCO. In both ovulatory and anovulatory PCO, the proportion of early growing (primary) follicles is significantly increased with a reciprocal decrease in the proportion of primordial follicles compared to normal ovaries [47]. These differences are particularly striking in anovulatory PCO [47]. There is decreased atresia of follicles from PCO in culture compared to those from normal ovaries [47]. Theca cells from PCO have constitutive increases in activity of multiple steroidogenic enzymes [44]; it is postulated that a similar defect contributes to adrenal androgen excess in affected women [44,45].
Women with PCOS have defects in both peripheral, which reflects primarily skeletal muscle, and hepatic insulin action, as well as pancreatic β-cell dysfunction [8,48]. These abnormalities are independent of obesity, although obesity substantially worsens these parameters [8,15,49]. However, in certain populations of women with PCOS, such as those from Scandinavia, obesity per se seems to account for insulin resistance [50,51]. This finding suggests that there may be ethnic variations in the mechanism of insulin resistance in PCOS.
The molecular mechanisms of insulin resistance in PCOS differ from those in other common insulin resistant states, such as obesity and T2D [8]. Despite the phenotypic similarity between PCOS and the type A syndrome of extreme insulin resistance and hyperandrogenism [5,8], no mutations in the coding portion of insulin receptor gene have been found in PCOS [8]. Studies in isolated subcutaneous adipocytes have shown no differences in insulin receptor number or affinity compared to weight-comparable control women [49,52]. However, the presence of post-binding defect in insulin signaling in adipocytes of women with PCOS was suggested by significantly decreased sensitivity to insulin-mediated glucose transport [49,52]. The abundance of GLUT4 glucose transporters was decreased in one [53] but not in another study [54] of isolated subcutaneous adipocytes from affected women. Additionally, an enhanced lipolytic effect of catecholamines was found in isolated visceral adipocytes in PCOS, which could contribute to insulin resistance by increasing free fatty acids release directly into the portal circulation [55]. The opposite abnormality, resistance to catecholamine-induced lipolysis, was present in isolated subcutaneous abdominal adipocytes [56].
Constitutive increases in insulin receptor serine phosphorylation were found in receptors isolated from cultured skin fibroblasts and from skeletal muscle, a classic insulin target tissue, in PCOS [57]. The increase in insulin receptor serine phosphorylation was accompanied by a decrease in insulin receptor tyrosine kinase activity that is essential for normal insulin signal transduction [8,57]. Studies using serine kinase inhibitors have suggested that a serine kinase extrinsic to the receptor is responsible for the constitutive increase in insulin receptor serine phosphorylation [58]. Studies in vivo have demonstrated a post-receptor defect in IRS-1-mediated activation of phosphatidylinositol 3-kinase (PI3K) activity, an early step the insulin signaling cascade, in skeletal muscle biopsies in parallel with decreased insulin-mediated glucose uptake in PCOS [59]. This study supports a physiologically relevant role for defective insulin signaling in PCOS, since skeletal muscle is the major site of insulin-mediated glucose uptake [60].
Defects in insulin signaling persist in myotubes cultures established from skeletal muscle biopsies obtained in women with PCOS, including decreased IRS-1-associated PI3K activity [61], analogous to findings in skeletal muscle biopsies during in vivo euglycemic hyperinsulinemic clamp studies in affected women [59]. Decreased IRS-1-mediated PI3K activity is associated with increased phosphorylation of IRS-1 on serine 312, a regulatory site that inhibits signaling, in PCOS myotubes [61]. These observations suggest that increased serine phosphorylation of IRS-1 may contribute to insulin resistance in the major target tissue for glucose uptake [59,61].
In two studies, however, PCOS myotubes cultures established from affected women with documented insulin resistance in vivo [61,62] were no longer insulin resistant. Indeed, Corbould et al. [61] found that PCOS myotubes have increased basal and insulin-stimulated glucose transport. Increased abundance of the non-insulin regulated GLUT1 glucose transporter accounted for the increased rates of basal glucose uptake. In contrast, decreased insulin-stimulated glucose transport persisted in another study of PCOS myotubes [54] from insulin resistant affected women. The reason for the discrepancies between these studies in PCOS myotubes is unclear. Nevertheless, these observations suggest that both intrinsic abnormalities and extrinsic factors in the in vivo environment account for insulin resistance in PCOS myotubes [61].
The insulin resistance in skin fibroblasts [63] and skeletal muscle [64] in PCOS is selective, affecting metabolic, but not mitogenic signaling pathways. Further, there appear to be tissue-specific differences in insulin sensitivity in PCOS. Insulin action to stimulate androgen production is preserved in ovarian theca cells [43], while granulosa-lutein cells are resistant to insulin's action on glucose metabolism [65]. These findings may explain the paradox of the persistent reproductive actions of insulin in the face of metabolic insulin resistance in PCOS [8].
In skeletal muscle, there is intriguing evidence that activation of mitogenic insulin signaling pathways contributes to resistance to insulin's metabolic actions in PCOS. Both in skeletal muscle biopsies acutely isolated from the in vivo environment [64,66] as well as in long-term cultures of myotubes established from these biopsies [64], there is constitutive activation of the mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase (ERK) pathway in PCOS. Raf-1 abundance is increased and the activity of p21Ras is decreased suggesting that altered mitogenic signaling begins at the level of Raf-1. Pharmacologic inhibition of MEK1/2 reduces IRS-1 Serine 312 phosphorylation and increases IRS-1 association with the p85 subunit of PI3K in both PCOS and control women. This observation suggests that MAPK-ERK pathway contributes to the normal feedback inhibition of metabolic insulin signaling through the serine phosphorylation of IRS-1. Furthermore, constitutive activation of this serine kinase mitogenic pathway may contribute to metabolic insulin resistance in PCOS [64].
Serine phosphorylation of a key regulatory enzyme of androgen biosynthesis, cytochrome P450c17, which catalyzes both 17α-hydroxylase and C17,20 lyase activities, increased its C17,20 lyase activity [67]. This observation has led to the hypothesis that the same serine kinase contributes to insulin resistance through serine phosphorylation of the insulin receptor and, perhaps, IRS-1, as well as hyperandrogenism through serine phosphorylation of P450c17 [8,68]. However, attempts to prove this hypothesis have been unsuccessful to date [69]. Recently, the Rho-associated, coiled-coil containing protein kinase/Rho pathway was identified as a candidate pathway that can serine phosphorylate both P450c17 and the insulin receptor (Fig. 2) [70].
It has been long debated whether hyperandrogenemia causes insulin resistance or vice-versa. Studies in which insulin levels were lowered by diazoxide [71] or insulin sensitivity was improved by metformin [72] or the thiazolidinedione, troglitazone [73], showed that insulin can directly stimulate ovarian androgen production in PCOS. This action appears to be mediated by insulin acting through its cognate receptor [74] rather the spillover occupancy of IGF-1 receptors as previously thought [8]. However, this action is only seen at more physiologic insulin levels in the presence of LH suggesting that insulin acts more as a co-gonadotropin [43]. It has also been shown that insulin can stimulate adrenal androgen production by enhancing sensitivity to ACTH [75]. Insulin does not affect ovarian function in normal women [76] suggesting that pre-existing polycystic ovarian changes, such as theca cell hyperplasia, are essential for insulin-mediated ovarian hyperandrogenism. In addition, lowering insulin levels ameliorates, but does not abolish, hyperandrogenism suggesting the coexistence of other contributing factors.
Insulin signaling in the brain is also important for the control of reproduction as well as body weight in animal models [8]. The selective central nervous system insulin receptor female knockout mouse develops disrupted LH release, impaired folliculogenesis and obesity [77]. Furthermore, targeted disruption of insulin and leptin receptors in hypothalamic and pituitary pro-opiomelanocortin neurons results in hyperandrogenemia, peripheral insulin resistance and reduced fertility in female mice [78]. Conversely, global disruption of the pituitary insulin receptor [79] protects against infertility and increases in LH release that occur in female mice with diet-induced obesity. These studies in female mice with diet-induced obesity [79,80] indicate that insulin signaling in the pituitary and ovary are preserved, despite resistance to insulin action in metabolic tissue. This finding is another example of tissue-specific differences in insulin action that may contribute to the reproductive phenotype of PCOS. The observation that insulin sensitizing drugs can restore ovulation in women with PCOS [8,81] is consistent with a role for central nervous system insulin sensitivity in the control of human reproduction, although alterations in gonadal steroid feedback or peripheral insulin levels may also contribute to the ovulation-inducing effects of insulin sensitizing drugs [81].
Women with upper-body obesity, who have many features of MetS, often have increased androgen production [82,83]. Higher levels of endogenous androgens are associated with increased risk for MetS in women [84]. Androgens are independent predictors of MetS in PCOS [17,18]. It has been hypothesized that androgens are a common final path for these metabolic defects in PCOS and upper-body obesity [85].
Administration of testosterone to female-to-male transsexuals to achieve levels in the normal male range results in decreased insulin-mediated glucose uptake [86] and increased visceral fat mass [87]. In normal postmenopausal women, administration of a weak synthetic androgen also increases visceral fat [88]. Consistent with these observations, blocking androgen action with a receptor antagonist in women with PCOS can improve insulin sensitivity, visceral adiposity, and dyslipidemia [89,90]. Additional factors contribute to insulin resistance, since suppression of androgens improves but does not completely restore normal insulin sensitivity in PCOS [91, 92]. Nevertheless, these findings indicate that there are adverse and reversible metabolic actions of androgens in women.
The role of androgens in the pathogenesis of PCOS has received renewed attention over the past 15 years [35,41]. The landmark studies of Dumesic et al. [41] have shown that prenatal androgen exposure can reproduce the reproductive and metabolic features of PCOS in non-human primates. Similar effects of prenatal androgens are seen in other species, such as sheep [41]. In humans, the putative source of androgens would be the fetal ovary or adrenal since the placenta is an effective barrier to maternal androgens excess [8]. Human experiments of nature support the hypothesis that fetal androgen excess, secondary to congenital adrenal hyperplasia or androgen-secreting neoplasms, can permanently alter LH secretion [45]. Androgen levels have not been elevated in cord blood from female offspring of women with PCOS in two studies [39,93], while a third study did find increased testosterone levels using a less specific hormone assay [94]. However, these findings do not preclude a role for intrauterine androgen excess earlier in gestation as the fetal ovary does express the enzymes required for androgen biosynthesis, P450c17, as early as the second trimester [95]. It is also possible that androgen exposure at later developmental windows during childhood and puberty programs features of PCOS. Elevated testosterone levels have been found in pubertal daughters of women with PCOS [40].
Evidence for a genetic susceptibility to PCOS is provided by well-documented familial clustering of PCOS, with to 40% of reproductive age sisters affected with hyperandrogenemia [35,37]. Twin studies have shown a correlation of 0.71 between monozygotic twins and 0.38 between dizygotic twins for PCOS [36] suggesting a major influence of genetic factors. Although some studies have suggested that there is an autosomal dominant mode of inheritance, these studies have been limited by a lack of prospective design, a failure to examine many first-degree relatives, and an unknown phenotype, except in reproductive age women [37]. PCOS is more likely a complex genetic disease with at least several major susceptibility genes [96].
Hyperandrogenemia is the major underlying reproductive phenotype in PCOS families and this finding has been replicated by other investigators [97,98]. There are two affected phenotypes in sisters of reproductive age: 1) classic PCOS with hyperandrogenemia and oligomenorrhea and 2) hyperandrogenemia with regular menses. Brothers of women with PCOS have elevations in the adrenal androgen, dehydroepiandrosterone sulfate [99], a marker of male androgen excess since testicular androgen production is tightly regulated by testosterone feedback on the hypothalamus [100]. This observation suggests that they have the same defect in androgen biosynthesis as their proband sisters with PCOS [99].
Affected sisters with either of the hyperandrogenemia phenotypes have insulin resistance [101], other MetS risk factors and elevated LDL levels [19]. Our studies indicate that mothers and brothers also have elevated LDL levels and evidence for insulin resistance [102]. Therefore, reproductive and metabolic abnormalities track together in PCOS families suggesting that they may reflect variation in the same gene or in closely linked genes, be causally related, or have a common pathogenesis. Since PCOS is a heterogeneous disorder, there may be genetic mechanisms other than hyperandrogenemia and non-genetic (e.g., environmental) factors that result in the PCOS phenotype. However, some of the phenotypic heterogeneity of PCOS appears to reflect variable expression of the same gene since several reproductive phenotypes can occur within family members who would be expected to share the same genetic basis for the disorder [35].
Most genetic analyses of PCOS have used candidate gene approaches. These are hypothesis-based studies where genes are selected because of prior evidence implicating them in disease risk [103] and, as a consequence, are limited by known biology. Given the diverse reproductive and metabolic disruptions that are features of PCOS, there is a wide range of biologic pathways from which to select candidate genes [104]. Mutational analyses of the insulin receptor gene were negative in PCOS, despite phenotypic similarity to the syndromes of hyperandrogenism and extreme insulin resistance [8]. Linkage studies implicating the gene encoding cholesterol side change cleavage enzyme, CYP11a, and the insulin gene VNTR could not be replicated in larger studies [8,37]. Case-control association studies of more than 150 candidate genes for PCOS have been limited by small sample size, phenotypic heterogeneity due to differences in diagnostic criteria, potential population stratification, confounding associated disorders such as obesity, limited examination of gene variants within each candidate gene, lack of replication and failure to control adequately for multiple testing [8,37]. Thus, the vast majority of genes implicated as associated with PCOS in these studies require further validation.
We have used the transmission disequilibrium test (TDT), a type of family-based association testing to map PCOS susceptibility variants employing a candidate gene approach [104]. The TDT tests for association in the presence of linkage using parent-affected-child trios to examine transmitted and non-transmitted parental alleles obviating the need for multiplex families [105]. We found strong evidence by TDT that an allele of a dinucleotide repeat D19S884 on chromosome 19p13.2 was linked and associated with the PCOS reproductive phenotype [37]. These findings were replicated in an independent sample of PCOS families and in a case-control study [8,37]. D19S884 is a microsatellite marker that had been selected for mapping the insulin receptor but it mapped to intron 55 of the fibrillin-3 (FBN3) gene located approximately1 Mb centromeric to the insulin receptor gene on chromosome 19p13.2 [96]. The FBN3 PCOS susceptibility allele is also associated with evidence for insulin resistance in women with PCOS and for pancreatic β-cell dysfunction in brothers, suggesting a sex difference in the associated metabolic phenotypes [96]. Fibrillin-3 shares sequence and structural similarity with fibrillin-1, which is mutated in Marfan syndrome [37,96]. Fibrillins are extracellular matrix molecules that modulate the bioavailability of members of the TGFβ signaling family. Members of this family are important in regulating reproductive and metabolic pathways [8]. The expression of fibrillin-3 is decreased in PCO [106].
The human HapMap contains common haplotypes and single nucleotide polymorphisms (SNPs) that identify these haplotypes, so-called tag SNPs [107]. Genome-wide association studies (GWAS) map the genome by genotyping these tag SNPs [107]. Population stratification can be controlled for in GWAS by the by adjusting for axes of ancestry-specific variation [8]. GWAS permit an unbiased interrogation of the entire genome for novel disease susceptibility loci and are, unlike candidate gene approaches, hypothesis generating [103]. The first PCOS GWAS [108] was in Han Chinese PCOS cases diagnosed by the Rotterdam criteria. There was strong evidence for association between PCOS and loci on chromosomes 2p16.3, 2p21 and 9q33.3. A second study by the same investigators [109] reported findings on a total sample of 8,226 Han Chinese PCOS cases and 7,578 controls confirmed the original three loci and identified eight new loci at 9q22.32, 11q22.1, 12q13.2, 12q14.3, 16q12.1, 19p13.3, 20q13.2, and a second independent signal at 2p16.3.
These loci contain several high priority candidate genes for PCOS, such as the genes encoding the LH, FSH, and insulin receptors, as well as genes associated in previous GWAS with T2D, THADA and HMGA2 [8]. As expected, new potential candidate genes for PCOS were also identified in pathways regulating transcription, chromatin structure, and cell growth, all plausible pathways for PCOS. Known genes located nearby the most significant SNP at 2p16.3 are GTF2A1L and LHCGR. GTF2A1L (TFIIA-alpha and beta-like factor) is germ cell-specific and a highly expressed in adult testis. LHCGR encodes the receptor for LH and human chorionic gonadotropin. The second SNP at 2p16.3 maps to the FSHR gene, which encodes the FSH receptor, another highly plausible candidate gene for PCOS given its characteristic abnormalities of folliculogenesis. The most significant SNP at 2p21 is in the THADA gene originally identified in thyroid adenomas but associated with T2D in a European GWAS [108]. The SNP at 9q33.3 is located within the DENND1A gene, which encodes a domain differentially expressed in normal and neoplastic cells (DENN) that can bind to and negatively regulate endoplasmic reticulum aminopeptidase-1.
The most significant SNP at 9q22.32 is located in an intron of the C9orf3 gene, which encodes C9orf3 protein, a member of M1 zinc aminopeptidase family. The SNP at 11q22.1 maps to an intron of the YAP1 gene, which encodes the YAP1 protein that is a transcriptional regulator critical in the Hippo signaling pathway. The most significant signal at 12q13.2 maps to an intragenic region between the RAB5B and SUOX genes, a region implicated in type 1 diabetes susceptibility. The most significant SNP at 12q14.3 maps to an intron of the HMGA2 gene, which encodes a transcription factor associated with a number of traits including T2D. The nearest gene to the signal at 16q12.1 is the TOX3 gene, which encodes the TOX3 protein, a member of the high-mobility-group proteins involved in chromatin structure. At 19p13.3, the most significant SNP maps to an intron of the INSR gene, which encodes the insulin receptor, a long-standing high priority candidate gene for PCOS. The most significant signal at 20q13.2 maps to an intragenic region between the SUMO1P1 and ZNF217 genes.
Two of the GWAS signals in Han Chinese PCOS were confirmed in large, well-designed studies in PCOS women of European origin that contained both discovery and replication cohorts [110,111]. The finding that the same genetic variants contribute to PCOS susceptibility in Chinese and European populations suggests that these susceptibility variants were present in an ancestral population prior to migration out of Africa [8]. Both studies replicated the association between DENND1A and PCOS [110,111]. The first study [110] also replicated the association between THADA and PCOS. The second study [111] found a significant association between one DENND1A variant and testosterone levels suggesting that this trait most likely had a genetic basis, consistent with the findings of Legro et al. [35]. Furthermore, the classic PCOS phenotype of hyperandrogenism and irregular menses but not PCO was associated another DENND1A variant [111] suggesting that there is a genetic susceptibility to the NICHD but not to the Rotterdam PCOS phenotype.
In summary, pathways linking metabolic cues with reproductive status are highly evolutionarily conserved being present in insects, flatworms, birds and mammals [8]. The recognition that the common reproductive disorder, PCOS, was associated with insulin resistance focused attention on elucidating the mechanisms of this association in humans [8]. This research has led to the discovery that insulin is also a reproductive hormone and that insulin signaling in the central nervous system is important for normal fertility. Moreover, androgens have adverse effects on insulin sensitivity and body fat distribution that contribute to the metabolic features of PCOS. Androgens may also play a role in the pathogenesis of PCOS through programming actions at critical developmental windows.
The reproductive and metabolic features of PCOS cluster in male as well as female relatives of affected women suggesting genetic susceptibility to these traits. Recent analyses using candidate gene as well as agnostic approaches have identified a number of confirmed disease susceptibility loci. These loci contain genes that are obvious high priority candidates, such as the receptors for LH, FSH, and insulin. Loci containing genes implicating new pathways in disease pathogenesis have also been mapped. In conclusion, the study of mechanisms linking reproduction and metabolism in PCOS has provided invaluable insight into novel actions of insulin and androgens, which has been translated into a new therapeutic approach with insulin sensitizing drugs. Furthermore, genetic analyses have implicated additional biologic pathways that may mediate this association (Fig. 3).
Figures and Tables
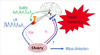 | Fig. 1Pathophysiology of the polycystic ovary syndrome (PCOS). The frequency of pulsatile gonadotropin-releasing hormone (GnRH) release is increased, which selectively increases LH secretion, while simultaneously suppressing follicle-stimulating hormone (FSH) release. Luteinizing hormone (LH) stimulates ovarian theca cell testosterone (T) production. There are also constitutive increases in the activity of multiple steroidogenic enzymes in polycystic ovaries contributing to increased androgen production. A similar defect is postulated to be present in the adrenal gland, which shares these steroidogenic enzymes, contributing to adrenal androgen excess that often occurs in PCOS (not shown). T is incompletely aromatized into estradiol by the adjacent granulosa cells because of relative FSH deficiency. Accordingly, increased circulating T levels are the result of increased LH-mediated T production, intrinsic increases in thecal T biosynthesis and decreased conversion of T into estradiol. T acts in the periphery to produce signs of androgen excess, such as hirsutism, acne and alopecia. T and androstenedione can also be aromatized extragonadally to estradiol and estrone, respectively, resulting in unopposed estrogen action on the endometrium (not shown). T feeds back on the hypothalamus to decrease the sensitivity to the normal feedback effects of estradiol and progesterone to slow GnRH pulse frequency. Insulin resistance is commonly associated with PCOS. Used with permission Andrea Dunaif. |
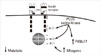 | Fig. 2Molecular mechanisms of insulin resistance in polycystic ovary syndrome (PCOS). The number and binding affinity of the insulin receptor is unchanged in PCOS, but there is a post-binding defect in insulin signaling resulting in marked decreases in insulin sensitivity in classic target tissues, such as muscle and adipocytes. There is a more modest defect in the maximal responsiveness to insulin of glucose uptake. The signaling defect appears to be due to increased inhibitory serine phosphorylation of the insulin receptor and IRS-1 secondary to intracellular serine kinases. This abnormality results in a selective decrease in insulin-mediated IRS-1-associated PI3K activation and resistance to insulin's metabolic action to stimulate glucose uptake. However, mitogenic signaling is preserved and there is constitutive activation of kinases in this MAPK-ERK mitogenic pathway in PCOS. Furthermore, it appears that kinases in this pathway feedback to decrease metabolic signaling by causing inhibitory serine phosphorylation of IRS-1. This feedback is increased in PCOS because this mitogenic serine pathway is constitutively activated. Serine phosphorylation of P450c17 increases its activity and it has been postulated that the same kinase may inhibit insulin signaling and increase androgen production in PCOS. P, phosphate; S, serine; S-S, disulfide bond; Y, tyrosine. Used with permission Andrea Dunaif. |
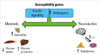 | Fig. 3Hypothesis for the Association of Reproduction and Metabolism in polycystic ovary syndrome (PCOS). Hyperinsulinemia resulting from insulin resistance can amplify the reproductive abnormalities of PCOS. Insulin signaling in the central nervous system is important for normal fertility. Androgens can produce insulin resistance and increase visceral fat. Susceptibility genes for PCOS may lead to increased androgen production and defects in insulin signaling. LH, luteinizing hormone. Used with permission Andrea Dunaif. |
References
1. Morgani GB. De Sedibus et Causis Morborum per Anatomen Indagata [The seats and causes of diseases investigated by anatomy]. 1765. Padua: Remondini.
2. Achard C, Theirs J. Le Virilisme pilaire et son association a l'insuffisance glycolytique (diabete des femme a barbe). Bull Acad Natl Med. 1921. 86:51–83.
3. Vague J. La différentiation sexuelle. Facteur determinant des formes de l'obesité. Presse Med. 1947. 55:339–340.
4. Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976. 294:739–745.
5. Taylor SI, Cama A, Accili D, Barbetti F, Quon MJ, de la Luz Sierra M, Suzuki Y, Koller E, Levy-Toledano R, Wertheimer E, Moncadaj VY, Kadowaki H, Kadowaki T. Mutations in the insulin receptor gene. Endocr Rev. 1992. 13:566–595.
6. Semple RK, Savage DB, Cochran EK, Gorden P, O'Rahilly S. Genetic syndromes of severe insulin resistance. Endocr Rev. 2011. 32:498–514.
7. Garg A. Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011. 96:3313–3325.
8. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012. In press.
9. Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980. 50:113–116.
10. Flier JS, Eastman RC, Minaker KL, Matteson D, Rowe JW. Acanthosis nigricans in obese women with hyperandrogenism. Characterization of an insulin-resistant state distinct from the type A and B syndromes. Diabetes. 1985. 34:101–107.
11. Dunaif A, Hoffman AR, Scully RE, Flier JS, Longcope C, Levy LJ, Crowley WF Jr. Clinical, biochemical, and ovarian morphologic features in women with acanthosis nigricans and masculinization. Obstet Gynecol. 1985. 66:545–552.
12. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998. 83:3078–3082.
13. Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999. 84:4006–4011.
14. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004. 89:2745–2749.
15. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989. 38:1165–1174.
16. O'Meara NM, Blackman JD, Ehrmann DA, Barnes RB, Jaspan JB, Rosenfield RL, Polonsky KS. Defects in beta-cell function in functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993. 76:1241–1247.
17. Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005. 90:1929–1935.
18. Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006. 91:492–497.
19. Sam S, Legro RS, Bentley-Lewis R, Dunaif A. Dyslipidemia and metabolic syndrome in the sisters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005. 90:4797–4802.
20. Ehrmann DA, Kasza K, Azziz R, Legro RS. PCOS/Troglitazone Study Group. Troglitazone Study Group: Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005. 90:66–71.
21. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F. National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005. 112:2735–2752.
22. Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002. 87:1017–1023.
23. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999. 84:165–169.
24. Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999. 22:141–146.
25. Gambineri A, Patton L, Altieri P, Pagotto U, Pizzi C, Manzoli L, Pasquali R. Polycystic ovary syndrome is a risk factor for type 2 diabetes: results from a long-term prospective study. Diabetes. 2012. 61:2369–2374.
26. Randeva HS, Tan BK, Weickert MO, Lois K, Nestler JE, Sattar N, Lehnert H. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012. In press.
27. Herzog AG. Polycystic ovarian syndrome in women with epilepsy: epileptic or iatrogenic? Ann Neurol. 1996. 39:559–560.
28. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010. 8:41.
29. Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol. 1998. 51:581–586.
30. Puurunen J, Piltonen T, Morin-Papunen L, Perheentupa A, Järvelä I, Ruokonen A, Tapanainen JS. Unfavorable hormonal, metabolic, and inflammatory alterations persist after menopause in women with PCOS. J Clin Endocrinol Metab. 2011. 96:1827–1834.
31. Schmidt J, Landin-Wilhelmsen K, Brännström M, Dahlgren E. Cardiovascular disease and risk factors in PCOS women of postmenopausal age: a 21-year controlled follow-up study. J Clin Endocrinol Metab. 2011. 96:3794–3803.
32. Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002. 87:2013–2017.
33. Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health: National Heart, Lung, and Blood Institute sponsored Women's Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008. 93:1276–1284.
34. Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010. 95:2038–2049.
35. Legro RS, Driscoll D, Strauss JF 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998. 95:14956–14960.
36. Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006. 91:2100–2104.
37. Urbanek M. The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007. 3:103–111.
38. Maliqueo M, Echiburú B, Crisosto N, Amigo P, Aranda P, Sánchez F, Sir-Petermann T. Metabolic parameters in cord blood of newborns of women with polycystic ovary syndrome. Fertil Steril. 2009. 92:277–282.
39. Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010. 95:2180–2186.
40. Sir-Petermann T, Codner E, Pérez V, Echiburú B, Maliqueo M, Ladrón de Guevara A, Preisler J, Crisosto N, Sánchez F, Cassorla F, Bhasin S. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009. 94:1923–1930.
41. Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007. 8:127–141.
42. Marshall JC, Eagleson CA. Neuroendocrine aspects of polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999. 28:295–324.
43. Franks S, Gilling-Smith C, Watson H, Willis D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin North Am. 1999. 28:361–378.
44. Nelson VL, Legro RS, Strauss JF 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999. 13:946–957.
45. Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev. 1995. 16:322–353.
46. Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000. 85:4047–4052.
47. Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008. 14:367–378.
48. Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. 1995. 96:520–527.
49. Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992. 41:1257–1266.
50. Holte J, Bergh T, Berne C, Wide L, Lithell H. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1995. 80:2586–2593.
51. Ovesen P, Moller J, Ingerslev HJ, Jorgensen JO, Mengel A, Schmitz O, Alberti KG, Moller N. Normal basal and insulin-stimulated fuel metabolism in lean women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1993. 77:1636–1640.
52. Ciaraldi TP, el-Roeiy A, Madar Z, Reichart D, Olefsky JM, Yen SS. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab. 1992. 75:577–583.
53. Rosenbaum D, Haber RS, Dunaif A. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Physiol. 1993. 264(2 Pt 1):E197–E202.
54. Ciaraldi TP, Aroda V, Mudaliar S, Chang RJ, Henry RR. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J Clin Endocrinol Metab. 2009. 94:157–163.
55. Ek I, Arner P, Rydén M, Holm C, Thörne A, Hoffstedt J, Wahrenberg H. A unique defect in the regulation of visceral fat cell lipolysis in the polycystic ovary syndrome as an early link to insulin resistance. Diabetes. 2002. 51:484–492.
56. Ek I, Arner P, Bergqvist A, Carlström K, Wahrenberg H. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab. 1997. 82:1147–1153.
57. Dunaif A, Xia J, Book CB, Schenker E, Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest. 1995. 96:801–810.
58. Li M, Youngren JF, Dunaif A, Goldfine ID, Maddux BA, Zhang BB, Evans JL. Decreased insulin receptor (IR) autophosphorylation in fibroblasts from patients with PCOS: effects of serine kinase inhibitors and IR activators. J Clin Endocrinol Metab. 2002. 87:4088–4093.
59. Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS). Am J Physiol Endocrinol Metab. 2001. 281:E392–E399.
60. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988. 37:667–687.
61. Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A, Dunaif A. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005. 288:E1047–E1054.
62. Eriksen M, Pørneki AD, Skov V, Burns JS, Beck-Nielsen H, Glintborg D, Gaster M. Insulin resistance is not conserved in myotubes established from women with PCOS. PLoS One. 2010. 5:e14469.
63. Book CB, Dunaif A. Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1999. 84:3110–3116.
64. Corbould A, Zhao H, Mirzoeva S, Aird F, Dunaif A. Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome. Diabetes. 2006. 55:751–759.
65. Rice S, Christoforidis N, Gadd C, Nikolaou D, Seyani L, Donaldson A, Margara R, Hardy K, Franks S. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod. 2005. 20:373–381.
66. Rajkhowa M, Brett S, Cuthbertson DJ, Lipina C, Ruiz-Alcaraz AJ, Thomas GE, Logie L, Petrie JR, Sutherland C. Insulin resistance in polycystic ovary syndrome is associated with defective regulation of ERK1/2 by insulin in skeletal muscle in vivo. Biochem J. 2009. 418:665–671.
67. Zhang LH, Rodriguez H, Ohno S, Miller WL. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1995. 92:10619–10623.
68. Bremer AA, Miller WL. The serine phosphorylation hypothesis of polycystic ovary syndrome: a unifying mechanism for hyperandrogenemia and insulin resistance. Fertil Steril. 2008. 89:1039–1048.
69. Martens JW, Geller DH, Arlt W, Auchus RJ, Ossovskaya VS, Rodriguez H, Dunaif A, Miller WL. Enzymatic activities of P450c17 stably expressed in fibroblasts from patients with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000. 85:4338–4346.
70. Tee MK, Dong Q, Miller WL. Pathways leading to phosphorylation of p450c17 and to the posttranslational regulation of androgen biosynthesis. Endocrinology. 2008. 149:2667–2677.
71. Nestler JE, Barlascini CO, Matt DW, Steingold KA, Plymate SR, Clore JN, Blackard WG. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1989. 68:1027–1032.
72. Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism. 1994. 43:647–654.
73. Dunaif A, Scott D, Finegood D, Quintana B, Whitcomb R. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996. 81:3299–3306.
74. Willis D, Franks S. Insulin action in human granulosa cells from normal and polycystic ovaries is mediated by the insulin receptor and not the type-I insulin-like growth factor receptor. J Clin Endocrinol Metab. 1995. 80:3788–3790.
75. Moghetti P, Castello R, Negri C, Tosi F, Spiazzi GG, Brun E, Balducci R, Toscano V, Muggeo M. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metab. 1996. 81:881–886.
76. Nestler JE, Singh R, Matt DW, Clore JN, Blackard WG. Suppression of serum insulin level by diazoxide does not alter serum testosterone or sex hormone-binding globulin levels in healthy, nonobese women. Am J Obstet Gynecol. 1990. 163(4 Pt 1):1243–1246.
77. Burks DJ, Font de Mora J, Schubert M, Withers DJ, Myers MG, Towery HH, Altamuro SL, Flint CL, White MF. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000. 407:377–382.
78. Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Brüning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010. 11:286–297.
79. Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010. 12:295–305.
80. Wu S, Divall S, Wondisford F, Wolfe A. Reproductive tissues maintain insulin sensitivity in diet-induced obesity. Diabetes. 2012. 61:114–123.
81. Dunaif A. Drug insight: insulin-sensitizing drugs in the treatment of polycystic ovary syndrome: a reappraisal. Nat Clin Pract Endocrinol Metab. 2008. 4:272–283.
82. Evans DJ, Hoffmann RG, Kalkhoff RK, Kissebah AH. Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J Clin Endocrinol Metab. 1983. 57:304–310.
83. Kirschner MA, Samojlik E, Drejka M, Szmal E, Schneider G, Ertel N. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab. 1990. 70:473–479.
84. Patel SM, Ratcliffe SJ, Reilly MP, Weinstein R, Bhasin S, Blackman MR, Cauley JA, Sutton-Tyrrell K, Robbins J, Fried LP, Cappola AR. Higher serum testosterone concentration in older women is associated with insulin resistance, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2009. 94:4776–4784.
85. Kissebah AH, Peiris AN. Biology of regional body fat distribution: relationship to non-insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1989. 5:83–109.
86. Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab. 1994. 79:265–271.
87. Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab. 1997. 82:2044–2047.
88. Lovejoy JC, Bray GA, Bourgeois MO, Macchiavelli R, Rood JC, Greeson C, Partington C. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women: a clinical research center study. J Clin Endocrinol Metab. 1996. 81:2198–2203.
89. Diamanti-Kandarakis E, Mitrakou A, Raptis S, Tolis G, Duleba AJ. The effect of a pure antiandrogen receptor blocker, flutamide, on the lipid profile in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1998. 83:2699–2705.
90. Gambineri A, Patton L, Vaccina A, Cacciari M, Morselli-Labate AM, Cavazza C, Pagotto U, Pasquali R. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab. 2006. 91:3970–3980.
91. Dunaif A, Green G, Futterweit W, Dobrjansky A. Suppression of hyperandrogenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1990. 70:699–704.
92. Moghetti P, Tosi F, Castello R, Magnani CM, Negri C, Brun E, Furlani L, Caputo M, Muggeo M. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab. 1996. 81:952–960.
93. Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, Newnham JP, Hart R. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab. 2009. 94:3714–3720.
94. Barry JA, Kay AR, Navaratnarajah R, Iqbal S, Bamfo JE, David AL, Hines M, Hardiman PJ. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010. 30:444–446.
95. Cole B, Hensinger K, Maciel GA, Chang RJ, Erickson GF. Human fetal ovary development involves the spatiotemporal expression of p450c17 protein. J Clin Endocrinol Metab. 2006. 91:3654–3661.
96. Urbanek M, Sam S, Legro RS, Dunaif A. Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab. 2007. 92:4191–4198.
97. Kashar-Miller M, Azziz R. Heritability and the risk of developing androgen excess. J Steroid Biochem Mol Biol. 1999. 69:261–268.
98. Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003. 88:2031–2036.
99. Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002. 87:2134–2138.
100. Veldhuis JD, Rogol AD, Samojlik E, Ertel NH. Role of endogenous opiates in the expression of negative feedback actions of androgen and estrogen on pulsatile properties of luteinizing hormone secretion in man. J Clin Invest. 1984. 74:47–55.
101. Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab. 2002. 87:2128–2133.
102. Sam S, Legro RS, Essah PA, Apridonidze T, Dunaif A. Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2006. 103:7030–7035.
103. Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005. 6:95–108.
104. Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, Strauss JF 3rd, Spielman RS, Dunaif A. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A. 1999. 96:8573–8578.
105. Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet. 1993. 52:506–516.
106. Jordan CD, Bohling SD, Charbonneau NL, Sakai LY. Fibrillins in adult human ovary and polycystic ovary syndrome: is fibrillin-3 affected in PCOS? J Histochem Cytochem. 2010. 58:903–915.
107. International HapMap Consortium. A haplotype map of the human genome. Nature. 2005. 437:1299–1320.
108. Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Li Z, You L, Zhao J, Liu J, Liang X, Zhao X, Sun Y, Zhang B, Jiang H, Zhao D, Bian Y, Gao X, Geng L, Li Y, Zhu D, Sun X, Xu JE, Hao C, Ren CE, Zhang Y, Chen S, Zhang W, Yang A, Yan J, Ma J, Zhao Y. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011. 43:55–59.
109. Shi Y, Zhao H, Cao Y, Yang D, Li Z, Zhang B, Liang X, Li T, Chen J, Shen J, Zhao J, You L, Gao X, Zhu D, Zhao X, Yan Y, Qin Y, Li W, Yan J, Wang Q, Geng L, Ma J, Zhao Y, He G, Zhang A, Zou S, Yang A, Liu J, Li B, Wan C, Shi J, Yang J, Jiang H, Xu JE, Qi X, Sun Y, Zhang Y, Hao C, Ju X, Zhao D, Ren CE, Li X, Zhang W, Zhang J, Wu D, Zhang C, He L, Chen ZJ. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012. 44:1020–1025.
110. Goodarzi MO, Jones MR, Li X, Chua AK, Garcia OA, Chen YD, Krauss RM, Rotter JI, Ankener W, Legro RS, Azziz R, Strauss JF 3rd, Dunaif A, Urbanek M. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet. 2012. 49:90–95.
111. Welt CK, Styrkarsdottir U, Ehrmann DA, Thorleifsson G, Arason G, Gudmundsson JA, Ober C, Rosenfield RL, Saxena R, Thorsteinsdottir U, Crowley WF, Stefansson K. Variants in DENND1A are associated with polycystic ovary syndrome in women of European ancestry. J Clin Endocrinol Metab. 2012. 97:E1342–E1347.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download