Abstract
The natural history of primary hyperparathyroidism, due to parathyroid adenoma, is unknown. Furthermore, spontaneous resolution of parathyroid necrosis or hemorrhage is rare and usually asymptomatic. Here, we report a case of parathyroid apoplexy of primary hyperparathyroidism, presenting as auditory hallucinations, accompanied with hypocalcemia. A 39-year-old man who was incidentally diagnosed with primary hyperparathyroidism, and waiting surgery for parathyroidectomy presented to psychiatric service with auditory hallucinations. He developed tetany, while taking psychiatric drugs. On a follow-up investigation, his serum calcium level fell from 11.8 to 5.8 mg/dL. His intact parathyroid hormone level also decreased from 1,017 pg/mL to 71.1 pg/mL. The parathyroid apoplexy was confirmed after a surgical removal of the infarcted adenoma. The auditory hallucinations disappeared, and serum calcium level was returned to within the normal range.
Primary hyperparathyroidism is one of the most common endocrine disorders. Primary hyperparathyroidism causes hypercalcemia through excessive secretion of parathyroid hormone (PTH) due to adenoma, hyperplasia, or less commonly, carcinoma of the parathyroid glands. It is most often caused by a parathyroid adenoma. The possible signs and symptoms vary, and the clinical course is diverse. Moreover, neurologic and neuropsychiatric symptoms are especially difficult to identify and investigate. Psychiatric symptoms reported in hyperparathyroidism include depression, anxiety, psychosis, lethargy, apathy, delirium, and dementia [1]. An incidence of psychopathology at 4% out of 405 cases has been reported in a literature review on hyperparathyroidism [2]. Surgical removal of parathyroid adenoma is recommended for most symptomatic patients. However, the natural history and biochemical and hormonal changes of primary hyperparathyroidism due to parathyroid adenoma are not well elucidated. Previously reported cases that presented with hypocalcemia resulted from necrosis of the adenoma and incompetence of the remaining parathyroid glands to produce the necessary amounts of PTH or hypercalcemia due to excessive PTH release, followed by finally normocalcemia [3,4].
Here, we report a rare case of parathyroid apoplexy of primary hyperparathyroidism presenting as auditory hallucinations accompanied with hypocalcemia.
A 39-year-old man underwent a physical examination in September 2009 and an incidental neck mass was found by a neck ultrasound sonogram (USG) in him. He was referred to our hospital for further evaluation. He had no symptoms, but his previous medical history included acute pancreatitis 2 years earlier, which was thought to be due to small quantities of alcohol ingestion. Calcium level was not measured at that time. He did not smoke and had an unremarkable family history. He also had no episode of renal stone or peptic ulcer. Neck examination revealed a palpable mass on the lower portion of the right part of the anterior neck. His physical examination was otherwise unremarkable. At that time, primary hyperparathyroidism was diagnosed with total serum calcium of 11.8 mg/dL (normal range, 8.6-10.2 mg/dL), phosphate of 2.3 mg/dL (normal range, 2.7-4.5 mg/dL), and intact PTH of 1,017 pg/mL (normal range, 15-65 pg/mL) (Table 1). There were no other abnormal laboratory findings. Ultrasonography revealed a hypoechoic mass of about 1.72 × 1.74 × 2.71 cm surrounded by a hyperechogenic wall in the patient's right inferior thyroid (Fig. 1A). Neck computed tomography (CT) showed a low-density mass of approximately 1.5 × 2.4 cm in the same area, which was considered to be a parathyroid adenoma (Fig. 1B). A dual energy X-ray absorption scan was also performed, which detected no bone loss. A parathyroid scan demonstrated a right inferior parathyroid adenoma (Fig. 2). Chest roentgenogram and electrocardiogram (ECG) revealed no abnormalities. He was put on the waiting list for surgery, which was a long delay in our hospital. Moreover, the patient had postponed the surgery for personal reasons.
Four months later and still before surgery, the patient developed insomnia and auditory hallucinations. He heard imaginary voices calling his name and therefore visited the psychiatric clinic in January 2010. He was treated with the followings antipsychotic drugs for approximately 14 weeks: 25 mg quetiapine, 6 mg risperidone, 1.5 mg lorazepam, 5 mg procyclidine HCl, 30 mg propranolol. His insomnia and auditory hallucinations improved slightly, but were not completely resolved.
On April 18, 2010, the patient visited the emergency room (ER) with tetany. Recently, lethargy and weakness was also noted. He had experienced paresthesia in the hands, face and limbs, and he experienced an episode of hand muscle spasm one day prior to admission. Chvostek's sign and Trousseau's sign were observed when he visited the ER. The results of other physical examinations were similar to those of previous tests. There was no pain in the parathyroid area, and ultrasonographic evaluations were serially performed (Fig. 3). The size of the suspected parathyroid adenoma was about 1.85 × 1.55 × 2.57 cm and showed similar to the result of the previous thyroid USG study (Fig. 3A). However, neck CT showed a mass of approximately 1.8 × 2.9 cm on the inferior and posterior aspects of the right lobe (Fig. 3B). The internal low-density lesion was enlarged, and fine needle aspiration of this mass was performed. A cytological examination showed a few macrophages and neutrophils as well as numerous necrotic cells. Unexpectedly, the patient's serum calcium level decreased to 5.8 mg/dL (Table 1). Intact PTH level also decreased to 71.1 pg/mL compared with the previous measurement and phosphorous was 3.4 mg/mL. The QT interval of his ECG was at the upper limit of the normal range (430 msec).
Severe symptomatic tetany is the indication for emergent correction of hypocalcemia. The patient was therefore treated with intravenous calcium followed by oral calcium and 1, 25-dihydroxyvitamin D supplementation. Initially, 2 g calcium gluconate (1 g = 93 mg elemental calcium) were mixed in 100 mL of 5% dextrose water and administrated for approximately 15 minutes. Next, continuous clalcium was replaced to provide about 1.0 mg/kg/hr of elemental calcium, and was adjusted to maintain the serum calcium concentration at the lower end of the normal range. In addition, 0.25 µg calcitriol which has a rapid onset of action was treated twice daily, and 2 g calcium gluconate t.i.d. was also given during the transition from intravenous to oral therapy. After 5 days, the intravenous infusion was gradually tapered as the oral therapy took effect. He had no side effects, including nausea and constipation, and his neuromuscular symptoms of acute hypocalcemia gradually disappeared.
He underwent conventional surgery on May 2, 2010. A parathyroid adenoma was found in the right inferior pole of the thyroid, and the specimen grossly measured 3.2 × 2.0 × 1.5 cm (Fig. 4). Final pathologic findings were consistent with proliferation of parathyroid cells, the presence of fibrotic tissue with hemosiderin deposition, and a cavity surrounded by fibrous walls containing macrophages, lymphocytes, multinucleated giant cells, and cell debris (Fig. 5). No swelling was seen on other glands. It was compatible with autoinfarction of parathyroid adenoma. Postoperatively, the patient has remained in eucalcemia with oral calcium and vitamin D replacement followed by reducing, this was reduced over time. The psychiatric symptoms also significantly improved.
Primary hyperparathyroidism is an endocrine disorder in which one or more of the four parathyroid glands secrete excess PTH, resulting in hypercalcemia. Single gland adenoma is the most common cause (75-85%), while multi-gland adenoma arises in a substantial proportion (two glands in 2-12% of cases, three glands in < 1-2%, and four or more in < 1-15%), and parathyroid carcinoma is rare (≤ 1%) [5]. Lower pole adenomas of the thyroid are more common than are upper pole adenomas and; sizes (range, 1-3 cm) [5]. Most patients with primary hyperparathyroidism have no obvious symptoms or signs of disease, with their disease detected by an incidental finding of hypercalcemia. Symptoms and clinical signs often relate to chronic hypercalcemia rather than to increased PTH levels [6]. Clinical presentations associated with hypercalcemia include gastrointestinal distress, peripheral neuromuscular complaints, nephrolithiasis, osteoporosis, and psychiatric manifestations.
Psychiatric symptoms include lethargy, fatigue, depression, memory loss, psychosis, ataxia, delirium, and coma [1]. Although these symptoms are of concern, they are difficult to quantify, and there is debate about whether they are directly attributable to the underlying disease. Therefore, the incidence of psychiatric symptoms from primary hyperparathyroidism varies from 4% to 57% depending on the report [2,7,8]. In a report by Petersen [7], among 54 patients with hyperparathyroidism who were examined for psychiatric features, 36 had affective disturbance, 12 had impaired memory, and 5 had acute or organic psychosis. Of the patients in that study 37% experienced moderate personality changes, 21% had severe personality changes, and 9% were psychotic [7]. Hallucinations have been rarely reported [9]. In our patient, auditory hallucinations occurred. Unfortunately, when he visited a psychiatric clinic, his calcium level was not measured. However, it is considered that he had prolonged exposure to hypercalcemia while waiting for his operation. The association between a disturbed calcium balance with hypercalcemia and psychiatric manifestation is known, but the underlying mechanisms have not been fully clarified. Increased calcium is thought to affect neurologic function because psychiatric changes improve if hypercalcemia resolves after parathyroidectomy. However, a linear association between serum calcium or PTH and psychiatric abnormalities does not necessarily exist [7,10]. Apathy and depression may be related to the role of calcium in enhancing the release of the biosynthetic enzyme dopamine β-hydroxylase from neuronal granules [11]. Serum PTH may exert a direct central nervous system (CNS) effect, however, the mediation is still thought to derive from increased CNS levels of calcium [12,13].
The natural history of parathyroid adenoma is not well known. In addition, spontaneous remission of primary hyperparathyroidism has been rarely reported. The only cure for primary hyperparathyroidism is surgical removal of a parathyroid adenoma or adenomas. Left untreated, primary hyperparathyroidism is usually associated with morbidity. Hemorrhage or infarction in the parathyroid adenoma leading to the spontaneous remission of hyperparathyroidism was first described by Norris [14] in 1946, but this has not yet been reported in our country. In a recent review of the literature, Wootten and Orzeck [4] performed a meta-analysis of 50 cases of autoparathyroidectomy. Acute and spontaneous extinction of chief cell adenomas is a well-characterized phenomenon that can be broken down into three etiologic categories: autoinfarction, acute extracapsular hemorrhage and acute intracapsular hemorrhage [4]. Nylen et al. [15] proposed that hemorrhage and infarction are two stages of the same phenomenon, which begins with necrosis and variable hemorrhage, the extent of which defines the clinical course. This condition was referred to as autoparathyroidectomy or parathyroid apoplexy due to similarities with pituitary apoplexy [16]. The development of tetany and hypocalcemia after spontaneous remission has been reported rarely in the literature, but was observed in the present case [17]. It is known and hypothesized to consist of three phases [16,17]. At first, massive release of PTH from a necrosed gland exacerbates the hyperclacemia, which is reflected by the worsening period of 1-2 weeks. The second phase is occasionally followed by potential acute hypocalcemia, especially in those patients with previous bone involvement because the recovery of these remaining glands takes several days. This phenomenon is called "hungry bone syndrome." In the third phase, remission is achieved and ultimately normocalcemia returns, albeit sometimes only partially. We suppose that the abrupt drop in serum PTH due to parathyroid apoplexy led to hypocalcemia and hungry bone syndrome in this patient. This condition was similar to that of hungry bone syndrome following parathyroidectomy. The fluctuations in the intact PTH and serum calcium level of the patient were extensive (from 1,017 to 71.1 pg/mL, from 11.8 to 5.8 mg/dL, respectively). The rise in ALP (574 IU/L; normal range, 35-129 IU/L) reflects increased bone turnover. Therefore, the sudden withdrawal of PTH causes to increase in bone uptake of calcium for bone remodeling, and such patients may experience hypocalcemia.
The pathophysiology of this disease remains unknown. In a review of the literature by Kovacs and Gay [18], seven of eight clinical cases had adenomas larger than 2 cm in diameter at the time of pathologic analysis of the specimen. This measurement was taken after infarction. The presence of a large, hyperfunctioning gland that outgrows its blood supply is a plausible explanation and leads to parathyroid ischemia [18,19]. In the present case, the size of the parathyroid adenoma was more than 3 cm (gross size, 3.2 × 2.0 × 1.5 cm). Nevertheless, our patient did not preoperatively experience the severe neck pain by parathyroid ischemia, making this case different from report of Govindaraj et al. [19]. A relatively large sized parathyroid adenoma may be susceptible to ischemia and necrosis. Therefore, early surgical intervention is indicated in these patients. In meta-analysis by Wootten and Orzeck [4], the average decrease in circulating levels of PTH was 69.1% upon spontaneous remission of primary hyperparathyroidism. This suggests that spontaneous remission was as effective as controlling the disease as a surgical procedure in most cases. Although parathyroid autoinfarction provides at least temporary control of hyperparathyroidism and its metabolic derangements, some cases were observed to relapse into hyperparathyroidism [4]. Surgical resection of parathyroid adenoma is required for cure.
In conclusion, we present a rare case of parathyroid apoplexy in primary hyperparathyroidism presented with auditory hallucination accompanied with hypocalcemia. Early diagnosis and follow-up of hypercalcemia can prevent unnecessary and potentially harmful treatments with antidepressants, anxiolytics, or antipsychotic drugs which can further suppress the psychiatric state of the patient. Additionally, it is important that the physician is aware of the subtle behavioral presentations of primary hyperparathyroidism. Furthermore, a large sized parathyroid adenoma may tend to cause spontaneous ischemia. If the patient is a surgical candidate and the size of the parathyroid adenoma is large, surgical management should be performed without delay.
Figures and Tables
Fig. 1
A. Thyroid ultrasound sonogram shows an approximately 1.72 × 1.74 × 2.71 cm-sized hypoechoic mass (arrow) surrounded by a hyperechogenic wall in the right inferior thyroid. B. Neck computed tomography shows an approximately 1.5 × 2.4 cm-sized mass (arrow) with internal low density on the inferior and posterior aspects of the right lobe at the enhanced phase.

Fig. 3
Follow-up images. A. Thyroid ultrasound sonogram shows an approximately 1.85 × 1.55 × 2.57 cm-sized hypoechoic mass (arrow) surrounded by hyperechogenic wall in the right inferior thyroid. B. Neck computed tomography shows an approximately 1.8 × 2.9 cm-sized mass (arrow) on the inferior and posterior aspects of the right lobe at the enhanced phase. The low-density lesion was enlarged.
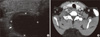
Fig. 4
Gross findings of the resected parathyroid gland. A, B. Parathyroid adenoma specimen of a 3.2 × 2.0 × 1.5 cm mass.

Fig. 5
Microscopic findings of parathyroid adenoma. A. Proliferative parathyroid cells, the presence of fibrous tissue with hemosiderin deposition (arrow), and a cavity surrounded by fibrous walls are shown (H&E stain, × 40). B. The remaining parathyroid adenoma (arrow) with fibrous capsule in the peripheral area (H&E stain, × 40).

References
1. Geffken GR, Ward HE, Staab JP, Carmichael SL, Evans DL. Psychiatric morbidity in endocrine disorders. Psychiatr Clin North Am. 1998. 21:473–489.
2. Feldman F, Mitchell W, Soll S. Psychosis, Cushing's syndrome and hyperparathyroidism. Can Med Assoc J. 1968. 98:508–511.
3. Efremidou EI, Papageorgiou MS, Pavlidou E, Manolas KJ, Liratzopoulos N. Parathyroid apoplexy, the explanation of spontaneous remission of primary hyperparathyroidism: a case report. Cases J. 2009. 2:6399.
4. Wootten CT, Orzeck EA. Spontaneous remission of primary hyperparathyroidism: a case report and meta-analysis of the literature. Head Neck. 2006. 28:81–88.
5. Fraser WD. Hyperparathyroidism. Lancet. 2009. 374:145–158.
6. Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999. 341:1249–1255.
7. Petersen P. Psychiatric disorders in primary hyperparathyroidism. J Clin Endocrinol Metab. 1968. 28:1491–1495.
8. Cope O. Hyperparathyroidism: diagnosis and management. Am J Surg. 1960. 99:394–403.
9. Joborn C, Hetta J, Palmér M, Akerström G, Ljunghall S. Psychiatric symptomatology in patients with primary hyperparathyroidism. Ups J Med Sci. 1986. 91:77–87.
10. Walker MD, McMahon DJ, Inabnet WB, Lazar RM, Brown I, Vardy S, Cosman F, Silverberg SJ. Neuropsychological features in primary hyperparathyroidism: a prospective study. J Clin Endocrinol Metab. 2009. 94:1951–1958.
11. Devaris DP, Mehlman I. Psychiatric presentations of endocrine and metabolic disorders. Prim Care. 1979. 6:245–265.
12. Guisado R, Arieff AI, Massry SG, Lazarowitz V, Kerian A. Changes in the electroencephalogram in acute uremia. Effects of parathyroid hormone and brain electrolytes. J Clin Invest. 1975. 55:738–745.
13. Bilezikian JP. Primary hyperparathyroidism. Drug Ther. 1982. 7:41–48.
14. Norris EH. Primary hyperparathyroidism; a report of five cases that exemplify special features of this disease (infarction of a parathyroid adenoma; oxyphil adenoma). Arch Pathol (Chic). 1946. 42:261–273.
15. Nylen E, Shah A, Hall J. Spontaneous remission of primary hyperparathyroidism from parathyroid apoplexy. J Clin Endocrinol Metab. 1996. 81:1326–1328.
16. Natsui K, Tanaka K, Suda M, Yasoda A, Yonemitsu S, Nakao K. Spontaneous remission of primary hyperparathyroidism due to hemorrhagic infarction in the parathyroid adenoma. Intern Med. 1996. 35:646–649.
17. Mir R, Gerold T, Khan S, Weitz J. Spontaneous infarction of parathyroid adenoma: case report and literature review. Head Neck. 1993. 15:566–568.
18. Kovacs KA, Gay JD. Remission of primary hyperparathyroidism due to spontaneous infarction of a parathyroid adenoma. Case report and review of the literature. Medicine (Baltimore). 1998. 77:398–402.
19. Govindaraj S, Wasserman J, Rezaee R, Pearl A, Bergman DA, Wang BY, Urken ML. Parathyroid adenoma autoinfarction: a report of a case. Head Neck. 2003. 25:695–699.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download